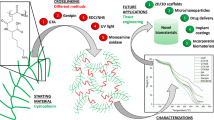
Abstract
Microtubes (MT) are one of the emerging biomaterials as they can play an important role as porous conduits, guidance cues, microvascular joining, and places where tissue composition matches microtubular geometry. In this regard, protein- and polymeric-based MT have reported biodegradable protein conduit for nerve repair and silk micro-vasculature-based repair of the blood vessel. Here in this article, we report for the first time the rapid production method of cerium oxide microtubes (CeO2-MT) and their cytocompatibility with C2C12 cells. The microtubes were prepared by precipitating CeO2 in an aqueous solution on the electrospun polycaprolactone (PCL) nanofibers, followed by thermal degradation at 440 °C for 5 h to remove the PCL, thereby obtaining the CeO2-MT. X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, Raman spectroscopy, and Brunauer–Emmett–Teller (BET) surface area measurements confirmed the presence of MT with the phase of CeO2, with fiber diameter ≈280 nm and high surface area due to tubular space. MT was cytocompatible with mouse myoblast cell line C2C12 cells and protected the cells from reactive oxygen species (ROS) insult at 100 µM H2O2 concentration. Therefore, the findings demonstrate the potentiality of MT tubular structure as novel biomaterials for scavenging high ROS and as a guided cell cue for potential muscle tissue regeneration.



Similar content being viewed by others
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
C. Mahapatra, R.K. Singh, J.H. Lee, J. Jung, J.K. Hyun, H.W. Kim, Nano-shape varied cerium oxide nanomaterials rescue human dental stem cells from oxidative insult through intracellular or extracellular actions. Acta Biomater. 50, 142–153 (2017). https://doi.org/10.1016/j.actbio.2016.12.014
M. You, K. Li, Y. Xie, L. Huang, X. Zheng, The effects of cerium valence states at cerium oxide coatings on the responses of bone mesenchymal stem cells and macrophages. Biol. Trace Elem. Res. 179, 259–270 (2017)
F. Wei, C.J. Neal, T.S. Sakthivel, T. Kean, S. Seal, M.J. Coathup, Multi-functional cerium oxide nanoparticles regulate inflammation and enhance osteogenesis. Mater. Sci. Eng., C 124, 112041 (2021)
A. Datta, S. Mishra, K. Manna, K.D. Saha, S. Mukherjee, S. Roy, Pro-oxidant therapeutic activities of cerium oxide nanoparticles in colorectal carcinoma cells. ACS Omega 5, 9714–9723 (2020)
L. Zeng, H. Cheng, Y. Dai, Z. Su, C. Wang, L. Lei, D. Lin, X. Li, H. Chen, K. Fan, S. Shi, In vivo regenerable cerium oxide nanozyme-loaded pH/H2O2-responsive nanovesicle for tumor-targeted photothermal and photodynamic therapies. ACS Appl. Mater. Interfaces. 13, 233–244 (2021)
B. Córdoba-Jover, A. Arce-Cerezo, J. Ribera, M. Pauta, D. Oró, G. Casals, G. Fernández-Varo, E. Casals, V. Puntes, W. Jiménez, M. Morales-Ruiz, Cerium oxide nanoparticles improve liver regeneration after acetaminophen-induced liver injury and partial hepatectomy in rats. J. Nanobiotechnol. 17, 112 (2019)
J.-W. Kim, C. Mahapatra, J.-Y. Hong, M.S. Kim, K.W. Leong, H.-W. Kim, J.K. Hyun, Functional recovery of contused spinal cord in rat with the injection of optimal-dosed cerium oxide nanoparticles. Adv. Sci. 4, 1700034 (2017)
A. Arya, N.K. Sethy, A. Gangwar, N. Bhargava, A. Dubey, M. Roy, G. Srivastava, S.K. Singh, M. Das, K. Bhargava, Cerium oxide nanozyme modulate the ‘exercise’ redox biology of skeletal muscle. Mater. Res. Express 4, 055401 (2017)
G.G. Genchi, A. Degl’Innocenti, A.R. Salgarella, I. Pezzini, A. Marino, A. Menciassi, S. Piccirillo, M. Balsamo, G. Ciofani, Modulation of gene expression in rat muscle cells following treatment with nanoceria in different gravity regimes. Nanomedicine. 13, 2821–2833 (2018)
I.-S. Park, C. Mahapatra, J.S. Park, K. Dashnyam, J.-W. Kim, J.C. Ahn, P.-S. Chung, D.S. Yoon, N. Mandakhbayar, R.K. Singh, J.-H. Lee, K.W. Leong, H.-W. Kim, Revascularization and limb salvage following critical limb ischemia by nanoceria-induced Ref-1/APE1-dependent angiogenesis. Biomater 242, 119919 (2020)
A. Jain, M. Behera, C. Mahapatra, N.R. Sundaresan, K. Chatterjee, Nanostructured polymer scaffold decorated with cerium oxide nanoparticles toward engineering an antioxidant and anti-hypertrophic cardiac patch. Mater. Sci. Eng. C 118, 111416 (2021)
F. Pagliari, C. Mandoli, G. Forte, E. Magnani, S. Pagliari, G. Nardone, S. Licoccia, M. Minieri, P. Di Nardo, E. Traversa, Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano 6, 3767–3775 (2012)
P.B. Sigler, B.J. Masters, The hydrogen peroxide-induced Ce*(III)-Ce(IV) exchange system1. J. Am. Chem. Soc. 79, 6353–6357 (1957)
A. Filippi, F. Liu, J. Wilson, S. Lelieveld, K. Korschelt, T. Wang, Y. Wang, T. Reich, U. Pöschl, W. Tremel, H. Tong, Antioxidant activity of cerium dioxide nanoparticles and nanorods in scavenging hydroxyl radicals. RSC Adv. 9, 11077–11081 (2019)
Y. Yang, Z. Mao, W. Huang, L. Liu, J. Li, J. Li, Q. Wu, Redox enzyme-mimicking activities of CeO2 nanostructures: intrinsic influence of exposed facets. Sci. Rep. 6, 35344 (2016)
M. Wang, H. He, D. Liu, M. Ma, Y. Zhang, Preparation, characterization and multiple biological properties of peptide-modified cerium oxide nanoparticles. Biomol. 12, 1277 (2022)
T. Naganuma, E. Traversa, The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomater. 35, 4441–4453 (2014)
S.K. Nethi, H.S. Nanda, T.W.J. Steele, C.R. Patra, Functionalized nanoceria exhibit improved angiogenic properties. J. Mater. Chem. B. 5, 9371–9383 (2017)
S. Das, S. Singh, J.M. Dowding, S. Oommen, A. Kumar, T.X.T. Sayle, S. Saraf, C.R. Patra, N.E. Vlahakis, D.C. Sayle, W.T. Self, S. Seal, The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomater. 33, 7746–7755 (2012)
E. Bazikyan, A. Chunikhin, Morphological and immunohistochemical effect of CeO2 nanoparticles on reparative osteogenesis of the jaw bones, (2021). https://doi.org/10.7324/JAPS.2021.120217
G.-M. Lyu, Y.-J. Wang, X. Huang, H.-Y. Zhang, L.-D. Sun, Y.-J. Liu, C.-H. Yan, Hydrophilic CeO2 nanocubes protect pancreatic β-cell line INS-1 from H2O2-induced oxidative stress. Nanoscale 8, 7923–7932 (2016)
G.D.O.C. Sathish Ponnurangam, Irina V. Chernyshova, Katherine Wood, Clark Tung-Hui Hung, and Ponisseril Somasundaran, Beneficial effects of cerium oxide nanoparticles in development of chondrocyte-seeded hydrogel constructs and cellular response to interleukin insults. Tissue Engineering Part A 20 (2014) 2908–2919.
M.J. Akhtar, M. Ahamed, H. Alhadlaq, Anti-inflammatory CeO2 nanoparticles prevented cytotoxicity due to exogenous nitric oxide donors via induction rather than inhibition of superoxide/nitric oxide in HUVE cells. Mol. 26, 5416 (2021)
M.B. Kolli, N.D.P.K. Manne, R. Para, S.K. Nalabotu, G. Nandyala, T. Shokuhfar, K. He, A. Hamlekhan, J.Y. Ma, P.S. Wehner, L. Dornon, R. Arvapalli, K.M. Rice, E.R. Blough, Cerium oxide nanoparticles attenuate monocrotaline induced right ventricular hypertrophy following pulmonary arterial hypertension. Biomater. 35, 9951–9962 (2014)
S.S. El Shaer, T.A. Salaheldin, N.M. Saied, S.M. Abdelazim, In vivo ameliorative effect of cerium oxide nanoparticles in isoproterenol-induced cardiac toxicity. Exp. Toxicol. Pathol. 69, 435–441 (2017)
G.G. Genchi, A. Degl’Innocenti, C. Martinelli, M. Battaglini, D. De Pasquale, M. Prato, S. Marras, G. Pugliese, F. Drago, A. Mariani, M. Balsamo, V. Zolesi, G. Ciofani, Cerium Oxide Nanoparticle Administration to Skeletal Muscle Cells under Different Gravity and Radiation Conditions. ACS Appl Mater Interfaces. 13(34), 40200–40213 (2021). https://doi.org/10.1021/acsami.1c14176
D. Lian, M.-M. Chen, H. Wu, S. Deng, X. Hu, The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxid. 11, 755 (2022)
M.-C. Chen, Y.-C. Sun, Y.-H. Chen, Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 9, 5562–5572 (2013)
M. Suhaeri, R. Subbiah, S.-H. Kim, C.-H. Kim, S.J. Oh, S.-H. Kim, K. Park, Novel platform of cardiomyocyte culture and coculture via fibroblast-derived matrix-coupled aligned electrospun nanofiber. ACS Appl. Mater. Interfaces. 9, 224–235 (2017)
H. Gao, J. Xiao, Y. Wei, H. Wang, H. Wan, S. Liu, Regulation of myogenic differentiation by topologically microgrooved surfaces for skeletal muscle tissue engineering. ACS Omega 6, 20931–20940 (2021)
K. Chun et al., An array of hollow microcapillaries for the controlled injection of genetic materials into animal/plant cells, Technical Digest. IEEE International MEMS 99 Conference. Twelfth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No.99CH36291), Orlando, FL, USA, 1999, pp. 406–411. https://doi.org/10.1109/MEMSYS.1999.746863
J. Li, Z. Liu, G. Huang, Z. An, G. Chen, J. Zhang, M. Li, R. Liu, Y. Mei, Hierarchical nanoporous microtubes for high-speed catalytic microengines. NPG Asia Mater. 6, e94–e94 (2014)
M. Lovett, C. Cannizzaro, L. Daheron, B. Messmer, G. Vunjak-Novakovic, D.L. Kaplan, Silk fibroin microtubes for blood vessel engineering. Biomater. 28, 5271–5279 (2007)
D. Sooriyaarachchi, Y. Zhou, S. Maharubin, G.Z. Tan, Microtube-embedded microfluidic devices for potential applications in blood brain barrier research. Procedia Manuf. 48, 294–301 (2020)
A. Oyane, K. Onuma, A. Ito, H.-M. Kim, T. Kokubo, T. Nakamura, Formation and growth of clusters in conventional and new kinds of simulated body fluids. J. Biomed. Mater. Res., Part A 64A, 339–348 (2003)
Y. Polyak, Z. Bastl, XPS and factor analysis study of initial stages of cerium oxide growth on polycrystalline tungsten. Surf. Interface Anal. 47, 663–671 (2015)
M. Comet, L. Schreyeck-Reinert, C. Louis, H. Fuzellier, Synthesis and characterization of high surface area aluminium and alumina microtubes from carbonaceous materials. J. Mater. Chem. 12, 754–757 (2002)
C. Schilling, A. Hofmann, C. Hess, M.V. Ganduglia-Pirovano, Raman spectra of polycrystalline CeO2: a density functional theory study. J. Phys. Chem. C 121, 20834–20849 (2017)
K. Li, Y. Xie, M. You, L. Huang, X. Zheng, Cerium oxide-incorporated calcium silicate coating protects MC3T3-E1 osteoblastic cells from H2O2-induced oxidative stress. Biol. Trace Elem. Res. 174, 198–207 (2016)
Y. Feng, Z. Xu, C. Peng, H. Huang, J. Hu, A facile route to obtain binary micro-nano roughness on composite coating surface. Eur. Phys. J. Appl. Phys. 82, 21302 (2018)
M. Aguirre, E. Johansson Salazar-Sandoval, M. Johansson, A. Ahniyaz, M. Paulis, J.R. Leiza, Hybrid acrylic/CeO2 nanocomposites using hydrophilic, spherical and high aspect ratio CeO2 nanoparticles. Journal of Materials Chemistry A. 2, 20280–20287 (2014). https://doi.org/10.1039/c4ta03620d
S. Polaka, P. Katare, B. Pawar, N. Vasdev, T. Gupta, K. Rajpoot, P. Sengupta, R.K. Tekade, Emerging ROS-modulating technologies for augmentation of the wound healing process. ACS. Omega. 7, 30657–30672 (2022)
H. Sadidi, S. Hooshmand, A. Ahmadabadi, S. Javad Hosseini, F. Baino, M. Vatanpour, S. Kargozar, Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules. 25(19):4559 (2020 ). https://doi.org/10.3390/molecules25194559
K. Min, O.-S. Kwon, A.J. Smuder, M.P. Wiggs, K.J. Sollanek, D.D. Christou, J.-K. Yoo, M.-H. Hwang, H.H. Szeto, A.N. Kavazis, S.K. Powers, Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J. Physiol. 593, 2017–2036 (2015)
S.A. Dogan, G. Giacchin, E. Zito, C. Viscomi, Redox signaling and stress in inherited myopathies. Antioxid Redox Signal. 37(4–6), 301–323 (2022). https://doi.org/10.1089/ars.2021.0266
S.K. Powers, A.J. Smuder, A.R. Judge, Oxidative stress and disuse muscle atrophy: cause or consequence? Current Opinion in Clinical Nutrition & Metabolic Care 15 (2012).
M.C. Gomez-Cabrera, C. Arc-Chagnaud, A. Salvador-Pascual, T. Brioche, A. Chopard, G. Olaso-Gonzalez, J. Viña, Redox modulation of muscle mass and function. Redox Biol. 35, 101531 (2020)
J. Kruk, B.H. Aboul-Enein, E. Duchnik, M. Marchlewicz, Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 72, 19 (2022)
H. Wu, H. Liao, F. Li, J. Lee, P. Hu, W. Shao, X. Li, D. Ling, Bioactive ROS-scavenging nanozymes for regenerative medicine: reestablishing the antioxidant firewall. Nano. Select. 1, 285–297 (2020)
F.M. Filetti, D.V. Vassallo, M. Fioresi, M.R. Simões, Reactive oxygen species impair the excitation-contraction coupling of papillary muscles after acute exposure to a high copper concentration. Toxicol. In Vitro 51, 106–113 (2018)
J.-W. Cao, S.-Y. Duan, H.-X. Zhang, Y. Chen, M. Guo, Zinc deficiency promoted fibrosis via ROS and TIMP/MMPs in the myocardium of mice. Biol. Trace Elem. Res. 196, 145–152 (2020)
J.-G. Lee, W.-J. Noh, H. Kim, M.-Y. Lee, Generation of reactive oxygen species contributes to the development of carbon black cytotoxicity to vascular cells Toxicological Research. Korean. Soc. Toxicol. 27, 161–166 (2011)
H.K. Choi, C.-H. Kim, S.N. Lee, T.-H. Kim, B.-K. Oh, Nano-sized graphene oxide coated nanopillars on microgroove polymer arrays that enhance skeletal muscle cell differentiation. Nano Convergence 8, 40 (2021)
C. Eren Cimenci, G. Uzunalli, O. Uysal, F. Yergoz, E. Karaca Umay, M.O. Guler, A.B. Tekinay, Laminin mimetic peptide nanofibers regenerate acute muscle defect. Acta Biomaterialia 60 , 190–200 (2017)
A. Cheesbrough, F. Sciscione, F. Riccio, P. Harley, L. R’Bibo, G. Ziakas, A. Darbyshire, I. Lieberam, W. Song, Biobased elastomer nanofibers guide light-controlled human-iPSC-derived skeletal myofibers. Adv. Mater. 34, 2110441 (2022)
M. Goldberg, R. Langer, X. Jia, Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 18, 241–268 (2007)
L.D. Ghosh, A. Jain, N.R. Sundaresan, K. Chatterjee, Elucidating molecular events underlying topography mediated cardiomyogenesis of stem cells on 3D nanofibrous scaffolds. Mat Sci Eng C-Mater 88, 104–114 (2018)
N. Hanib, F. Hamzah, Z. Omar, I. Subuki, Surface characterization on alkali-heat-treatment on titanium alloy. Malays. J. Anal. Sci. 20, 1429–1436 (2016)
F. Gentile, Time dependent adhesion of cells on nanorough surfaces. J. Biomech. 129, 110814 (2021)
Acknowledgements
The authors are thankful to the National Institute of Technology, Raipur (Chhattisgarh), India, and (CeNSE- MNCF) IISc Bangalore for providing support for this work. CM acknowledges the National Institute of Technology Raipur for the Seed Grant, Project No: NITRR/Seed Grant/2021-22/30. CM also gratefully acknowledged research support from the Department of Science and Technology, India, Science and Engineering Research Board (SERB), Sanction Order No SRG/2022/000348.
Author information
Authors and Affiliations
Contributions
C.M. conceived and designed the study. D.C. performed the experiments. A.K. and C.M analyzed the data and wrote first draft of the manuscript. All the authors reviewed the manuscript. C.M. prepared the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Highlights
1. Unique method for rapid production of cerium microtubes is reported.
2. Cerium oxide microtubes are cytocompatible with C2C12 cells.
3. Cerium oxide microtubes could provide directional cue to the C2C12 cells.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandra, D.K., Kumar, A. & Mahapatra, C. Rapid synthesis of novel cerium oxide microtubes and its cytocompatibility study. emergent mater. 6, 595–603 (2023). https://doi.org/10.1007/s42247-023-00498-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00498-9