Abstract
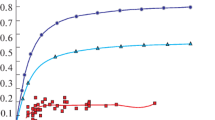
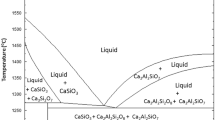
In order to increase the reaction rates between the molten steel and the slag and cut down the reduction time when the top slag of manganese ore is added into the molten steel, a method of directly alloying manganese ore has been experimented in a 500-kg induction furnace. The results show that the manganese yield is greater than 90% when the wire feeding method is used. The manganese yield is 43.26% within 1 min. In contrast, the manganese yield for the top-slag adding process is only 10.98% for the same duration. The mass transfer rate of the manganese is greater in the molten steel than in the slag, and the limiting factor is the mass transfer rate of the manganese in the slag in the period of 10−30 min. The slag composition area is closer to the area of high melting point for the wire feeding method than for the top-slag adding process. During the slagging process, refining slag composed of CaO and SiO2 is formed after 15 min; after 25−30 min, refining slag with a high basicity is formed and consists of CaO, SiO2 and Al2O3.













Similar content being viewed by others
References
W. Wu, S.F. Dai, P. Wang, D. Ma, B. Ni, Ironmak. Steelmak. 46 (2019) 469−476.
W. Wu, S.F. Dai, Y. Liu, J. Iron Steel Res. Int. 24 (2017) 908–915.
W. Wu, J.J. Gao, J.Q. Zeng, Y.H. Qi, J.C. Wang, X.D. Zhang, J. Iron Steel Res. Int. 23 (2016) 210–219.
L.N. Guo, J. Chen, M. Zhang, M. Liang, J. Iron Steel Res. Int. 19 (2012) No. 5, 1–8.
J. Chen, P.F. Tian, X.A. Song, N. Li, J.X. Zhou, J. Iron Steel Res. Int. 17 (2010) No. 3, 13–20.
S.F. Dai , W. Wu, D. Ma, X.D. Zhang, P. Wang, Iron and Steel 52 (2017) No. 8, 35–42.
T. Shimoo, S. Ando, H. Kimura, J. Jpn. Inst. Met. 48 (1984) 285–292.
T. Shimoo, S. Ando, H. Kimura, J. Jpn. Inst. Met. 48 (1984) 922–929.
K.D. Xu, G.C. Jiang, W.Z. Ding, L.P. Gu, S.Q. Guo, B.X. Zhao, ISIJ Int. 33 (1993) 104–108.
M. Ashizuka, A. Moribe, K. Sawamura, Tetsu-to-Hagane 61 (1975) 36–45.
D.Y. Kim, S.M. Jung, ISIJ Int. 56 (2016) 71–77.
N. Shinozaki, K. Ishido, K. Mori, Y. Kawai, Tetsu-to-Hagane 70 (1984) 73–80.
R. Kononov, O. Ostrovski, S. Ganguly, Metall. Mater. Trans. B 39 (2008) 662–668.
N. Anacleto, O. Ostrovski, S. Ganguly, ISIJ Int. 44 (2004) 1615–1622.
O.I. Ostrovski, T.J.M. Webb, ISIJ Int. 35 (1995) 1331–1339.
S.F. Dai, W. Wu, X.D. Zhang, P. Wang, China Metallurgy 27 (2017) No. 1, 12–18.
W. Wu, P. Wang, L. Lin, S.F. Dai, High Temperature Materials and Processes 37 (2018) 741–747.
C.F. Zhang, J.B. Chang, S.W. Li, Z.J. Han, D.G. Ma, S.X. Liu, Y.B. Xiang, Steelmaking 29 (2013) No. 6, 12–14.
C.F. Lv, D.L. Shang, Q. Sun, L. Kang, Z.Y. Qi, Steelmaking 31 (2015) No. 1, 40–43.
T. Takaoka, I. Sumi, Y. Kikuchi, Y. Kawai, ISIJ Int. 33 (1993) 98–103.
J. Safarian, Ø. Grong, L. Kolbeinsen, S.E. Olsen, ISIJ Int. 46 (2006) 1120–1129.
T. Matsuo, S. Fukagawa, T. Ikeda, Tetsu-to-Hagane 76 (1990) 1831–1838.
O.A. El Hady, A.E. Amer, I.S. El Mahallawi, Y.S. Shash, Mater. Sci. Forum 561–565 (2007) 85–89.
S.M. Jung, C.H. Rhee, D.J. Min, ISIJ Int. 42 (2002) 63–70.
E.T. Turkdogan, Fundamentals of steelmaking, The Institute of Materials Publishers, London, UK, 1996.
A. Sobandi, H.G. Katayama, T. Momono, ISIJ Int. 38 (1998) 953–958.
Acknowledgements
This research has been financially supported by the National Key R&D Program (2017YFB0304000) and the Beijing Natural Science Foundation (2172057) in China, the State Key Laboratory of Refractories and Metallurgy Foundation (G201804) and the National Natural Science Foundation of China (51704080, 51874102).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, W., Gao, Q., Zhang, B. et al. Reaction kinetics during direct alloying of manganese ore cored wire. J. Iron Steel Res. Int. 27, 282–294 (2020). https://doi.org/10.1007/s42243-020-00363-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-020-00363-7