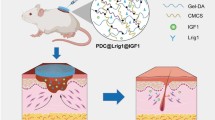
Abstract
Functionalized hydrogels stimulate the migration and morphogenesis of endothelial cells (ECs) and are useful substrates for wound healing. The present study investigates the feasibility of covalent conjugation of taurine (Tau) on a gelatin-based hydrogel. This hydrogel is expected to maintain positive charged growth factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factors (VEGFs) near ECs within the hydrogel microenvironment. The gelatin was conjugated with hydroxyl phenol (Ph) and Tau moieties, and in following that Ph residues were crosslinked through a horseradish peroxidase-catalyzed reaction. The migration characteristics of ECs were analyzed by scratch migration assay and microparticle-based cell migration assay. Cellular morphology and amounts of angiopoietin 1 (Ang 1), bFGF, and VEGF proteins were evaluated for encapsulated cells. The potential of synthesized hydrogels in wound healing was assessed by the percentage of reduction from the original wound size and histopathological analyses of rat skin. The incorporated Tau molecules within the hydrogel remained stable through covalent bonds during incubation. During extended incubation, the gelatin-based hydrogel conjugated with Tau improved the migration distance and number of existing migrated ECs. Immobilized Tau within the gelatin-based hydrogel induced high motility of ECs, accompanied by robust cytoskeleton extension and a cell subpopulation that expressed CD44 and CD31 receptors as well as enhancement of Ang 1, bFGF, and VEGF. We found that injectable Gel-Ph-Tau effectively improves wound-healing parameters.
Graphic abstract









Similar content being viewed by others
References
Dong RN, Guo BL (2021) Smart wound dressings for wound healing. Nano Today 41:101290. https://doi.org/10.1016/j.nantod.2021.101290
Masson-Meyers DS, TaM A, Caetano GF et al (2020) Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol 101(1–2):21–37. https://doi.org/10.1111/iep.12346
Farahani M, Shafiee A (2021) Wound healing: from passive to smart dressings. Adv Healthc Mater 10(16):e2100477. https://doi.org/10.1002/adhm.202100477
Liang YP, He JH, Guo BL (2021) Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 15(8):12687–12722. https://doi.org/10.1021/acsnano.1c04206
Zhang SH, Hou JY, Yuan QJ et al (2020) Arginine derivatives assist dopamine-hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem Eng J 392:123775. https://doi.org/10.1016/j.cej.2019.123775
Rodrigues M, Kosaric N, Bonham CA et al (2019) Wound healing: a cellular perspective. Physiol Rev 99(1):665–706. https://doi.org/10.1152/physrev.00067.2017
Han G, Ceilley R (2017) Chronic wound healing: a review of current management and treatments. Adv Ther 34(3):599–610. https://doi.org/10.1007/s12325-017-0478-y
Khanmohammadi M, Sakai S, Taya M (2019) Characterization of encapsulated cells within hyaluronic acid and alginate microcapsules produced via horseradish peroxidase-catalyzed crosslinking. J Biomat Sci-Polym E 30(4):295–307. https://doi.org/10.1080/09205063.2018.1562637
Wang P, Huang BS, Horng HC et al (2018) Wound healing. J Chin Med Assoc 2(81):94–101
Rieger KA, Birch NP, Schiffman JD (2013) Designing electrospun nanofiber mats to promote wound healing - a review. J Mater Chem B 1(36):4531–4541. https://doi.org/10.1039/c3tb20795a
Alven S, Aderibigbe B (2019) Combination therapy strategies for the treatment of malaria. Molecules 24(19):3601. https://doi.org/10.3390/molecules24193601
Opt Veld RC, van den Boomen OI, Lundvig DMS et al (2018) Thermosensitive biomimetic polyisocyanopeptide hydrogels may facilitate wound repair. Biomaterials 181(392):401. https://doi.org/10.1016/j.biomaterials.2018.07.038
Lin KS, Wang SY, Fan LJ et al (2017) Adipose-derived stem cells seeded in pluronic F-127 hydrogel promotes diabetic wound healing. J Surg Res 217:63–74. https://doi.org/10.1016/j.jss.2017.04.032
Zhu JM, Marchant RE (2011) Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devic 8(5):607–626. https://doi.org/10.1586/Erd.11.27
Catanzano O, Quaglia F, Boateng JS (2021) Wound dressings as growth factor delivery platforms for chronic wound healing. Expert Opin Drug Del 18(6):737–759. https://doi.org/10.1080/17425247.2021.1867096
Jo H, Yoon M, Gajendiran M et al (2020) Recent strategies in fabrication of gradient hydrogels for tissue engineering applications. Macromol Biosci 20(3):1900300. https://doi.org/10.1002/mabi.201900300
Hsu YY, Liu KL, Yeh HH et al (2019) Sustained release of recombinant thrombomodulin from cross-linked gelatin/hyaluronic acid hydrogels potentiate wound healing in diabetic mice. Eur J Pharm Biopharm 135:61–71. https://doi.org/10.1016/j.ejpb.2018.12.007
Dang LH, Huynh NT, Pham NO et al (2019) Injectable nanocurcumin-dispersed gelatin-pluronic nanocomposite hydrogel platform for burn wound treatment. B Mater Sci 42(2):71. https://doi.org/10.1007/s12034-019-1745-0
Khamrai M, Banerjee SL, Paul S et al (2019) Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: an eco-friendly sustainable synthesis method of wound healing patch. Int J Biol Macromol 122:940–953. https://doi.org/10.1016/j.ijbiomac.2018.10.196
Mansoori-Kermani A, Khalighi S, Akbarzadeh I et al (2022) Engineered hyaluronic acid-decorated niosomal nanoparticles for controlled and targeted delivery of epirubicin to treat breast cancer. Mater Today Bio 16:100349. https://doi.org/10.1016/j.mtbio.2022.100349
Singaravelu S, Ramanathan G, Raja MD et al (2016) Biomimetic interconnected porous keratin–fibrin–gelatin 3D sponge for tissue engineering application. Int J Biol Macromol 86:810–819. https://doi.org/10.1016/j.ijbiomac.2016.02.021
Benskin LL (2018) Evidence for polymeric membrane dressings as a unique dressing subcategory, using pressure ulcers as an example. Adv Wound Care 7(12):419–426. https://doi.org/10.1089/wound.2018.0822
Ye HL, Cheng JW, Yu K (2019) In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int J Biol Macromol 121:633–642. https://doi.org/10.1016/j.ijbiomac.2018.10.056
Ndlovu SP, Ngece K, Alven S et al (2021) Gelatin-based hybrid scaffolds: promising wound dressings. Polymers 13(17):2959. https://doi.org/10.3390/polym13172959
Dias JR, Baptista-Silva S, De Oliveira CMT et al (2017) In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur Polym J 95:161–173. https://doi.org/10.1016/j.eurpolymj.2017.08.015
Rath G, Hussain T, Chauhan G et al (2016) Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mat Sci Eng C Mater 58:242–253. https://doi.org/10.1016/j.msec.2015.08.050
Campiglio CE, Contessi Negrini N, Fare S et al (2019) Cross-linking strategies for electrospun gelatin scaffolds. Materials 12(15):2476. https://doi.org/10.3390/ma12152476
Badali E, Hosseini M, Mohajer M et al (2021) Enzymatic crosslinked hydrogels for biomedical application. Polym Sci Ser A 63(Suppl 1):S1–S22. https://doi.org/10.1134/S0965545x22030026
Khanmohammadi M, Dastjerdi MB, Ai A et al (2018) Horseradish peroxidase-catalyzed hydrogelation for biomedical applications. Biomater Sci 6(6):1286–1298. https://doi.org/10.1039/c8bm00056e
Davachi SM, Haramshahi SMA, Akhavirad SA et al (2022) Development of chitosan/hyaluronic acid hydrogel scaffolds via enzymatic reaction for cartilage tissue engineering. Mater Today Commun 30:103230. https://doi.org/10.1016/j.mtcomm.2022.103230
Badali E, Hosseini M, Varaa N et al (2022) Production of uniform size cell-enclosing silk derivative vehicles through coaxial microfluidic device and horseradish crosslinking reaction. Eur Polym J 172:111237. https://doi.org/10.1016/j.eurpolymj.2022.111237
Liu Y, Wong CW, Chang SW et al (2021) An injectable, self-healing phenol-functionalized chitosan hydrogel with fast gelling property and visible light-crosslinking capability for 3D printing. Acta Biomater 122:211–219. https://doi.org/10.1016/j.actbio.2020.12.051
Deng LL, Du CZ, Song PY et al (2021) The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Longev 2021:8852759. https://doi.org/10.1155/2021/8852759
Xu ZJ, Han SY, Gu ZP et al (2020) Advances and impact of antioxidant hydrogel in chronic wound healing. Adv Healthc Mater 9(5):1901502. https://doi.org/10.1002/adhm.201901502
Zdunska K, Dana A, Kolodziejczak A et al (2018) Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Phys 31(6):332–336. https://doi.org/10.1159/000491755
Farzamfar S, Naseri-Nosar M, Samadian H et al (2018) Taurine-loaded poly (ε-caprolactone)/gelatin electrospun mat as a potential wound dressing material: in vitro and in vivo evaluation. J Bioact Compat Pol 33(3):282–294. https://doi.org/10.1177/0883911517737103
Vittorazzi C, Endringer DC, De Andrade TU et al (2016) Antioxidant, antimicrobial and wound healing properties of Struthanthus vulgaris. Pharm Biol 54(2):331–337. https://doi.org/10.3109/13880209.2015.1040515
Li M, Chen J, Shi MT et al (2019) Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem Eng J 375:121999. https://doi.org/10.1016/j.cej.2019.121999
Qu J, Zhao X, Liang YP et al (2018) Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 183:185–199. https://doi.org/10.1016/j.biomaterials.2018.08.044
Lambert IH, Kristensen DM, Holm JB et al (2015) Physiological role of taurine–from organism to organelle. Acta Physiol 213(1):191–212. https://doi.org/10.1111/apha.12365
Goodarzi A, Khanmohammadi M, Ebrahimi-Barough S et al (2019) Alginate-based hydrogel containing taurine-loaded chitosan nanoparticles in biomedical application. Arch Neurosci 6(2):e86349. https://doi.org/10.5812/ans.86349
Khorani M, Bobe G, Matthews DG et al (2022) The impact of the hAPP695SW transgene and associated amyloid-β accumulation on murine hippocampal biochemical pathways. J Alzheimers Dis 85(4):1601–1619. https://doi.org/10.3233/Jad-215084
Khanmohammadi M, Sakai S, Taya M (2017) Impact of immobilizing of low molecular weight hyaluronic acid within gelatin-based hydrogel through enzymatic reaction on behavior of enclosed endothelial cells. Int J Biol Macromol 97:308–316. https://doi.org/10.1016/j.ijbiomac.2016.12.088
Wu L, Zhang QW, Li Y et al (2021) Collagen sponge prolongs taurine release for improved wound healing through inflammation inhibition and proliferation stimulation. Ann Transl Med 9(12):1010. https://doi.org/10.21037/atm-21-2739
Comino-Sanz IM, Lopez-Franco MD, Castro B et al (2021) The role of antioxidants on wound healing: a review of the current evidence. J Clin Med 10(16):3558. https://doi.org/10.3390/jcm10163558
Baek YY, Cho DH, Choe J et al (2012) Extracellular taurine induces angiogenesis by activating ERK-, Akt-, and FAK-dependent signal pathways. Eur J Pharmacol 674(2–3):188–199. https://doi.org/10.1016/j.ejphar.2011.11.022
Khoshfetrat AB, Khanmohammadi M, Sakai S et al (2016) Enzymatically-gellable galactosylated chitosan: hydrogel characteristics and hepatic cell behavior. Int J Biol Macromol 92:892–899. https://doi.org/10.1016/j.ijbiomac.2016.08.003
Khanmohammadi M, Sakai S, Ashida T et al (2016) Production of hyaluronic-acid-based cell-enclosing microparticles and microcapsules via enzymatic reaction using a microfluidic system. J Appl Polym Sci 133(16):43107. https://doi.org/10.1002/app.43107
Firouzi N, Khoshfetrat AB, Kazemi D (2020) Enzymatically gellable gelatin improves nano-hydroxyapatite-alginate microcapsule characteristics for modular bone tissue formation. J Biomed Mater Res A 108(2):340–350. https://doi.org/10.1002/jbm.a.36820
Wu M, Du Y, Liu YW et al (2014) Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways. PLoS ONE 9(3):e92857. https://doi.org/10.1371/journal.pone.0092857
Karimifard S, Rezaei N, Jamshidifar E et al (2022) pH-responsive chitosan-adorned niosome nanocarriers for co-delivery of drugs for breast cancer therapy. ACS Appl Nano Mater 5(7):8811–8825. https://doi.org/10.1021/acsanm.2c00861
Vernon RB, Sage EH (1999) A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res 57(2):118–133. https://doi.org/10.1006/mvre.1998.2122
Yang XM, Sarvestani SK, Moeinzadeh S et al (2013) Effect of CD44 binding peptide conjugated to an engineered inert matrix on maintenance of breast cancer stem cells and tumorsphere formation. PLoS ONE 8(3):e59147. https://doi.org/10.1371/journal.pone.0059147
Reno C, Marchuk L, Sciore P et al (1997) Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques 22(6):1082. https://doi.org/10.2144/97226bm16
Guo R, Xu SJ, Ma L et al (2011) The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen–chitosan dermal equivalents. Biomaterials 32(4):1019–1031. https://doi.org/10.1016/j.biomaterials.2010.08.087
Badali E, Goodarzi A, Khodayari H et al (2022) Layered dermal reconstitution through epigallocatechin 3-gallate loaded chitosan nanoparticle within enzymatically crosslinked polyvinyl alcohol/collagen fibrous mat. J Biomater Appl 37(3):502–516. https://doi.org/10.1177/08853282221104175
Salehi M, Zamiri S, Samadian H et al (2021) Chitosan hydrogel loaded with Aloe vera gel and tetrasodium ethylenediaminetetraacetic acid (EDTA) as the wound healing material: in vitro and in vivo study. J Appl Polym Sci 138(16):e50225. https://doi.org/10.1002/app.50225
Pardue EL, Ibrahim S, Ramamurthi A (2008) Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 4(4):203–214. https://doi.org/10.4161/org.4.4.6926
Chen WS, He SP, Xiang DM (2021) Hypoxia-induced retinal pigment epithelium cell-derived bFGF promotes the migration and angiogenesis of HUVECs through regulating TGF-β1/smad2/3 pathway. Cancer Lett 328(1):18–26. https://doi.org/10.1016/j.gene.2021.145695
Fagiani E, Christofori G (2013) Angiopoietins in angiogenesis. Cancer Lett 328(1):18–26. https://doi.org/10.1016/j.canlet.2012.08.018
Napoli S, Scuderi C, Gattuso G et al (2020) Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells 9(5):1511. https://doi.org/10.3390/cells9051151
Kandhwal M, Behl T, Singh S et al (2022) Role of matrix metalloproteinase in wound healing. Am J Transl Res 14(7):4391–4405
Tampa M, Georgescu SR, Mitran MI et al (2021) Current perspectives on the role of matrix metalloproteinases in the pathogenesis of basal cell carcinoma. Biomolecules 11(6):903. https://doi.org/10.3390/biom11060903
Author information
Authors and Affiliations
Contributions
FR, AG (Aida Goodarzi), and MK contributed to conceptualization; FN, NA, FR, SS, and SH contributed to formal analysis and investigation; NA, FN, and AG (Arash Goodarzi) contributed to writing—original draft preparation; AG (Arash Goodarzi) and MK contributed to supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All institutional and national guidelines for the care and use of laboratory animals were followed. Animal use and care were approved with National Ethics Committee of Fasa University of Medical Sciences (Ethical code: IR.FUMS.REC.1399.160) and were performed in accordance with the university’s guidelines. Furthermore, all animal experiments comply with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahimi, F., Ahmadkhani, N., Goodarzi, A. et al. Gelatin-based hydrogel functionalized with taurine moieties for in vivo skin tissue regeneration. Bio-des. Manuf. 6, 284–297 (2023). https://doi.org/10.1007/s42242-022-00227-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-022-00227-x