Abstract
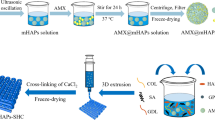
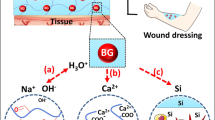
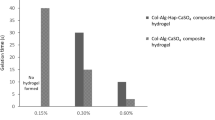
In this study, boron-doped hydroxyapatite (BHT)-loaded alginate/gelatin-based (A/G) hydrogel coating on Ti was fabricated to support bone integration through triggering osteoinduction, vascularization and immunomodulation. Initially, highly reproducible, cheap and time-effective BHT was produced, which significantly promoted higher osteogenic and angiogenic maturation, while a mild innate immune response was observed. The immense potential of BHT was evidenced by the production of a gap-filling A/G/BHT interphase on Ti implants to mimic the osseous extracellular matrix to achieve functional bridging and exert control over the course of innate immune response. We initially aminosilanized the implant surface using 3-aminopropyl triethoxysilane, and then coated it with 0.25% w/v alginate with 20 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide to allow the A/G/BHT pre-gel to disperse evenly and covalently attach on the surface. The pre-gel was added with 0.2 M NaCl to homogeneously blend BHT in the structure without inducing ionic crosslinking. Then, the coated implants were freeze-dried and stored. The coated layer demonstrated high cohesive and adhesive strength, and 8-month-long shelf-life at room temperature and normal humidity. The A/G/BHT was able to coat an irregularly shaped Ti implant. Osteoblasts and endothelial cells thrived on the A/G/BHT, and it demonstrated greatly improved osteogenic and angiogenic capacity. Moreover, A/G/BHT maintained macrophage viability and generated an acute increase in immune response that could be resolved rapidly. Finally, A/G/BHT was shown to induce the robust integration of implant in a rabbit femur osteochondral model within 2 months. Therefore, we concluded that A/G/BHT coatings could serve as a multifunctional reservoir, promoting the strong and rapid osseointegration of metallic implants.
Graphic abstract









Similar content being viewed by others
References
Rony L, Aguado E, Verlee B et al (2021) Microarchitecture of titanium cylinders obtained by additive manufacturing does not influence osseointegration in the sheep. Regen Biomater 8(4):rbab021. https://doi.org/10.1093/rb/rbab021
Prestat M, Thierry D (2021) Corrosion of titanium under simulated inflammation conditions: clinical context and in vitro investigations. Acta Biomater 136:72–87. https://doi.org/10.1016/j.actbio.2021.10.002
Lee H, Lee MK, Han G et al (2022) Customizable design of multiple-biomolecule delivery platform for enhanced osteogenic responses via ‘tailored assembly system.’ Bio-Des Manuf 5(3):451–464. https://doi.org/10.1007/s42242-022-00190-7
Koons GL, Diba M, Mikos AG (2020) Materials design for bone-tissue engineering. Nat Rev Mater 5(8):584–603. https://doi.org/10.1038/s41578-020-0204-2
Zhang XX, Lv Y, Lei Y et al (2021) Enhancement of corrosion resistance and biological performances of Cu-incorporated hydroxyapatite/TiO2 coating by adjusting Cu chemical configuration and hydroxyapatite contents. ACS Appl Bio Mater 4(1):903–917. https://doi.org/10.1021/acsabm.0c01390
Švagrová K, Horkavcová D, Jablonská E et al (2022) Titania-based sol-gel coatings with Ag, Ca-P applied on titanium substrate developed for implantation. J Biomed Mater Res B Appl Biomater 110(1):115–124. https://doi.org/10.1002/jbm.b.34895
Querido W, Rossi AL, Farina M (2016) The effects of strontium on bone mineral: a review on current knowledge and microanalytical approaches. Micron 80:122–134. https://doi.org/10.1016/j.micron.2015.10.006
Davis R, Singh A, Jackson MJ et al (2022) A comprehensive review on metallic implant biomaterials and their subtractive manufacturing. Int J Adv Manuf Technol 120(3–4):1473–1530. https://doi.org/10.1007/s00170-022-08770-8
Guo CY, Matinlinna JP, Tang AT (2012) Effects of surface charges on dental implants: past, present, and future. Int J Biomater 2012:381535. https://doi.org/10.1155/2012/381535
Silva-Bermudez P, Rodil S (2013) An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf Coat Technol 233:147–158. https://doi.org/10.1016/j.surfcoat.2013.04.028
Pazarçeviren AE, Tezcaner A, Evis Z (2021) Multifunctional natural polymer-based metallic implant surface modifications. Biointerphases 16(2):020803. https://doi.org/10.1116/6.0000876
Kuo YJ, Chen CH, Dash P et al (2022) Angiogenesis, osseointegration, and antibacterial applications of polyelectrolyte multilayer coatings incorporated with silver/strontium containing mesoporous bioactive glass on 316L stainless steel. Front Bioeng Biotechnol 10:818137. https://doi.org/10.3389/fbioe.2022.818137
Jariya SAI, Babu AA, Narayanan TSN et al (2022) Development of a novel smart carrier for drug delivery: ciprofloxacin loaded vaterite/reduced graphene oxide/PCL composite coating on TiO2 nanotube coated titanium. Ceramics Int 48(7):9579–9594. https://doi.org/10.1016/j.ceramint.2021.12.156
Moskalewicz T, Warcaba M, Łukaszczyk A et al (2022) Electrophoretic deposition, microstructure and properties of multicomponent sodium alginate-based coatings incorporated with graphite oxide and hydroxyapatite on titanium biomaterial substrates. Appl Surf Sci 575:151688. https://doi.org/10.1016/j.apsusc.2021.151688
Jing W, Feng L, Wang B et al (2021) Polymer-ceramic fiber nanocomposite coatings on titanium metal implant devices for diseased bone tissue regeneration. J Sci Adv Mater Dev 6(3):399–406. https://doi.org/10.1016/j.jsamd.2021.04.001
Mariani E, Lisignoli G, Borzì RM et al (2019) Biomaterials: foreign bodies or tuners for the immune response? Int J Mol Sci 20(3):636. https://doi.org/10.3390/ijms20030636
Szekalska M, Puciłowska A, Szymańska E et al (2016) Alginate: current use and future perspectives in pharmaceutical and biomedical applications. Int J Polym Sci 2016:17. https://doi.org/10.1155/2016/7697031
Tsou YH, Khoneisser J, Huang PC et al (2016) Hydrogel as a bioactive material to regulate stem cell fate. Bioact Mater 1(1):39–55. https://doi.org/10.1016/j.bioactmat.2016.05.001
Wang D, Zhu Y, Huang Y et al (2021) Pancreatic extracellular matrix/alginate hydrogels provide a supportive microenvironment for insulin-producing cells. ACS Biomater Sci Eng 7(8):3793–3805. https://doi.org/10.1021/acsbiomaterials.1c00269
Chan AW, Neufeld RJ (2009) Modeling the controllable pH-responsive swelling and pore size of networked alginate based biomaterials. Biomaterials 30(30):6119–6129. https://doi.org/10.1016/j.biomaterials.2009.07.034
Andersen T, Auk-Emblem P, Dornish M (2015) 3D cell culture in alginate hydrogels. Microarrays 4(2):133–161. https://doi.org/10.3390/microarrays4020133
Zhang X, Morits M, Jonkergouw C et al (2020) Three-dimensional printed cell culture model based on spherical colloidal lignin particles and cellulose nanofibril-alginate hydrogel. Biomacromolecules 21(5):1875–1885. https://doi.org/10.1021/acs.biomac.9b01745
Cavo M, Caria M, Pulsoni I et al (2018) A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo.” Sci Rep 8(1):5333. https://doi.org/10.1038/s41598-018-23250-4
Wang B, Díaz-Payno PJ, Browe DC et al (2021) Affinity-bound growth factor within sulfated interpenetrating network bioinks for bioprinting cartilaginous tissues. Acta Biomater 128:130–142. https://doi.org/10.1016/j.actbio.2021.04.016
Oh GW, Kim SC, Kim TH et al (2021) Characterization of an oxidized alginate-gelatin hydrogel incorporating a COS-salicylic acid conjugate for wound healing. Carbohydr Polym 252:117145. https://doi.org/10.1016/j.carbpol.2020.117145
Dodero A, Alberti S, Gaggero G et al (2021) An up-to-date review on alginate nanoparticles and nanofibers for biomedical and pharmaceutical applications. Adv Mater Interf 8(22):2100809. https://doi.org/10.1002/admi.202100809
Sarker B, Rompf J, Silva R et al (2015) Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int J Biol Macromol 78:72–78. https://doi.org/10.1016/j.ijbiomac.2015.03.061
Hasturk O, Jordan KE, Choi J et al (2020) Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 232:119720. https://doi.org/10.1016/j.biomaterials.2019.119720
Heltmann-Meyer S, Steiner D, Müller C et al (2021) Gelatin methacryloyl is a slow degrading material allowing vascularization and long-term use in vivo. Biomed Mater 16(6):065004. https://doi.org/10.1088/1748-605X/ac1e9d
Wang X, Ao Q, Tian X et al (2017) Gelatin-based hydrogels for organ 3D bioprinting. Polymers 9(9):401. https://doi.org/10.3390/polym9090401
Erkoc P, Uvak I, Nazeer MA et al (2020) 3D printing of cytocompatible gelatin-cellulose-alginate blend hydrogels. Macromol Biosci 20(10):e2000106. https://doi.org/10.1002/mabi.202000106
Ghanbari M, Salavati-Niasari M, Mohandes F (2021) Injectable hydrogels based on oxidized alginate-gelatin reinforced by carbon nitride quantum dots for tissue engineering. Int J Pharm 602:120660. https://doi.org/10.1016/j.ijpharm.2021.120660
Flores-Torres S, Peza-Chavez O, Kuasne H et al (2021) Alginate-gelatin-Matrigel hydrogels enable the development and multigenerational passaging of patient-derived 3D bioprinted cancer spheroid models. Biofabrication 13(2):025001. https://doi.org/10.1088/1758-5090/abdb87
Sun CK, Ke CJ, Lin YW et al (2021) Transglutaminase cross-linked gelatin-alginate-antibacterial hydrogel as the drug delivery-coatings for implant-related infections. Polymers 13(3):414. https://doi.org/10.3390/polym13030414
Chalitangkoon J, Wongkittisin M, Monvisade P (2020) Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int J Biol Macromol 159:194–203. https://doi.org/10.1016/j.ijbiomac.2020.05.061
Xue W, Liu B, Zhang H et al (2022) Controllable fabrication of alginate/poly-L-ornithine polyelectrolyte complex hydrogel networks as therapeutic drug and cell carriers. Acta Biomater 138:182–192. https://doi.org/10.1016/j.actbio.2021.11.004
Liu Y, Hu Q, Dong W et al (2022) Alginate/gelatin-based hydrogel with soy protein/peptide powder for 3D printing tissue-engineering scaffolds to promote angiogenesis. Macromol Biosci 22(4):e2100413. https://doi.org/10.1002/mabi.202100413
Pazarçeviren AE, Akbaba S, Tezcaner A et al (2022) Seamless and robust alginate/gelatin coating on Ti-6Al-4V as a gap filling interphase. Appl Surf Sci 581:152393. https://doi.org/10.1016/j.apsusc.2021.152393
Pazarçeviren AE, Tezcaner A, Keskin D et al (2021) Boron-doped biphasic hydroxyapatite/β-tricalcium phosphate for bone tissue engineering. Biol Trace Elem Res 199(3):968–980. https://doi.org/10.1007/s12011-020-02230-8
Çiftci Dede E, Korkusuz P, Bilgiç E et al (2022) Boron nano-hydroxyapatite composite increases the bone regeneration of ovariectomized rabbit femurs. Biol Trace Elem Res 200(1):183–196. https://doi.org/10.1007/s12011-021-02626-0
Chen S, Michálek M, Galusková D et al (2020) Multi-targeted B and Co co-doped 45S5 bioactive glasses with angiogenic potential for bone regeneration. Mater Sci Eng C Mater Biol Appl 112:110909. https://doi.org/10.1016/j.msec.2020.110909
Fu SY, Feng XQ, Lauke B et al (2008) Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Composites Part B Eng 39(6):933–961. https://doi.org/10.1016/j.compositesb.2008.01.002
Maji K, Dasgupta S, Bhaskar R et al (2020) Photo-crosslinked alginate nano-hydroxyapatite paste for bone tissue engineering. Biomed Mater 15(5):055019. https://doi.org/10.1088/1748-605X/ab9551
Carpentier G, Berndt S, Ferratge S et al (2020) Angiogenesis analyzer for ImageJ—a comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay.” Sci Rep 10(1):11568. https://doi.org/10.1038/s41598-020-67289-8
Otsuki B, Takemoto M, Fujibayashi S et al (2006) Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 27(35):5892–5900. https://doi.org/10.1016/j.biomaterials.2006.08.013
Brokesh AM, Gaharwar AK (2020) Inorganic biomaterials for regenerative medicine. ACS Appl Mater Interf 12(5):5319–5344. https://doi.org/10.1021/acsami.9b17801
Gizer M, Köse S, Karaosmanoglu B et al (2020) The effect of boron-containing nano-hydroxyapatite on bone cells. Biol Trace Elem Res 193(2):364–376. https://doi.org/10.1007/s12011-019-01710-w
Balasubramanian P, Hupa L, Jokic B et al (2017) Angiogenic potential of boron-containing bioactive glasses: in vitro study. J Mater Sci 52:8785–8792. https://doi.org/10.1007/s10853-016-0563-7
Li K, Lu X, Razanau I et al (2019) The enhanced angiogenic responses to ionic dissolution products from a boron-incorporated calcium silicate coating. Mater Sci Eng C Mater Biol Appl 101:513–520. https://doi.org/10.1016/j.msec.2019.04.009
Baniwal SK, Shah PK, Shi Y et al (2012) Runx2 promotes both osteoblastogenesis and novel osteoclastogenic signals in ST2 mesenchymal progenitor cells. Osteoporos Int 23(4):1399–1413. https://doi.org/10.1007/s00198-011-1728-5
Yahiro Y, Maeda S, Morikawa M et al (2020) BMP-induced Atoh8 attenuates osteoclastogenesis by suppressing Runx2 transcriptional activity and reducing the Rankl/Opg expression ratio in osteoblasts. Bone Res 8(1):32. https://doi.org/10.1038/s41413-020-00106-0
Chen D, Gong Y, Xu L et al (2019) Bidirectional regulation of osteogenic differentiation by the FOXO subfamily of Forkhead transcription factors in mammalian MSCs. Cell Prolif 52(2):e12540. https://doi.org/10.1111/cpr.12540
Duman E, Şahin Kehribar E, Ahan RE et al (2019) Biomineralization of calcium phosphate crystals controlled by protein-protein interactions. ACS Biomater Sci Eng 5(9):4750–4763. https://doi.org/10.1021/acsbiomaterials.9b00649
Shekaran A, Shoemaker JT, Kavanaugh TE et al (2014) The effect of conditional inactivation of beta 1 integrins using twist 2 Cre, Osterix Cre and osteocalcin Cre lines on skeletal phenotype. Bone 68:131–141. https://doi.org/10.1016/j.bone.2014.08.008
Kargozar S, Baino F, Hamzehlou S et al (2018) Bioactive glasses: sprouting angiogenesis in tissue engineering. Trends biotechnol 36(4):430–444. https://doi.org/10.1016/j.tibtech.2017.12.003
Westhauser F, Widholz B, Nawaz Q et al (2019) Favorable angiogenic properties of the borosilicate bioactive glass 0106-B1 result in enhanced in vivo osteoid formation compared to 45S5 Bioglass. Biomater Sci 7(12):5161–5176. https://doi.org/10.1039/c9bm01220f
Şen Ö, Emanet M, Çulha M (2019) Stimulatory effect of hexagonal boron nitrides in wound healing. ACS Appl Bio Mater 2(12):5582–5596. https://doi.org/10.1021/acsabm.9b00669
Raines AL, Berger MB, Patel N et al (2019) VEGF-A regulates angiogenesis during osseointegration of Ti implants via paracrine/autocrine regulation of osteoblast response to hierarchical microstructure of the surface. J Biomed Mater Res A 107(2):423–433. https://doi.org/10.1002/jbm.a.36559
Hu K, Olsen BR (2016) The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 91:30–38. https://doi.org/10.1016/j.bone.2016.06.013
Yu P, Zhang X, Liu N et al (2021) Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther 6(1):128. https://doi.org/10.1038/s41392-021-00507-5
Zhang R, Liu X, Xiong Z et al (2018) The immunomodulatory effects of Zn-incorporated micro/nanostructured coating in inducing osteogenesis. Artif Cells Nanomed Biotechnol 46(sup1):1123–1130. https://doi.org/10.1080/21691401.2018.1446442
Dollinger C, Ndreu-Halili A, Uka A et al (2017) Controlling incoming macrophages to implants: responsiveness of macrophages to gelatin micropatterns under M1/M2 phenotype defining biochemical stimulations. Adv Biosyst 1(6):1700041. https://doi.org/10.1002/adbi.201700041
Floquet CF, Sieben VJ, MacKay BA et al (2016) Determination of boron in produced water using the carminic acid assay. Talanta 150:240–252. https://doi.org/10.1016/j.talanta.2015.12.010
Su Y, Huang C, Lu F et al (2018) Alginate affects agglomeration state and uptake of 14C-labeled few-layer graphene by freshwater snails: implications for the environmental fate of graphene in aquatic systems. Environ Pollut 234:513–522. https://doi.org/10.1016/j.envpol.2017.11.087
Narayanan A, Kaur S, Peng C et al (2019) Viscosity attunes the adhesion of bioinspired low modulus polyester adhesive sealants to wet tissues. Biomacromolecules 20(7):2577–2586. https://doi.org/10.1021/acs.biomac.9b00383
Tiu BDB, Delparastan P, Ney MR et al (2019) Enhanced adhesion and cohesion of bioinspired dry/wet pressure-sensitive adhesives. ACS Appl Mater Interf 11(31):28296–28306. https://doi.org/10.1021/acsami.9b08429
Ratanavaraporn J, Chuma N, Kanokpanont S et al (2019) Beads fabricated from alginate, hyaluronic acid, and gelatin using ionic crosslinking and layer-by-layer coating techniques for controlled release of gentamicin. J Appl Polym Sci 136(1):46893. https://doi.org/10.1002/app.46893
Jing X, Sun Y, Liu Y et al (2021) Alginate/chitosan-based hydrogel loaded with gene vectors to deliver polydeoxyribonucleotide for effective wound healing. Biomater Sci 9(16):5533–5541. https://doi.org/10.1039/d1bm00911g
Reddy MSB, Ponnamma D, Choudhary R et al (2021) A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 13(7):1105. https://doi.org/10.3390/polym13071105
Zhang X, Wang K, Hu JY et al (2020) Role of a high calcium ion content in extending the properties of alginate dual-crosslinked hydrogels. J Mater Chem A 8(47):25390–25401. https://doi.org/10.1039/D0TA09315G
Davidenko N, Schuster CF, Bax DV et al (2016) Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry. J Mater Sci Mater Med 27(10):148. https://doi.org/10.1007/s10856-016-5763-9
Stubbe B, Mignon A, Declercq H et al (2019) Development of gelatin-alginate hydrogels for burn wound treatment. Macromol Biosci 19(8):e1900123. https://doi.org/10.1002/mabi.201900123
Hosseini S, Naderi-Manesh H, Vali H et al (2019) Contribution of osteocalcin-mimetic peptide enhances osteogenic activity and extracellular matrix mineralization of human osteoblast-like cells. Colloids Surf B Biointerf 173:662–671. https://doi.org/10.1016/j.colsurfb.2018.10.035
Moon YJ, Yun CY, Choi H et al (2016) Smad4 controls bone homeostasis through regulation of osteoblast/osteocyte viability. Exp Mol Med 48(9):e256. https://doi.org/10.1038/emm.2016.75
Tenkumo T, Vanegas Sáenz JR, Nakamura K et al (2018) Prolonged release of bone morphogenetic protein-2 in vivo by gene transfection with DNA-functionalized calcium phosphate nanoparticle-loaded collagen scaffolds. Mater Sci Eng C Mater Biol Appl 92:172–183. https://doi.org/10.1016/j.msec.2018.06.047
Yan J, Li J, Hu J et al (2018) Smad4 deficiency impairs chondrocyte hypertrophy via the Runx2 transcription factor in mouse skeletal development. J Biol Chem 293(24):9162–9175. https://doi.org/10.1074/jbc.RA118.001825
Liu YS, Liu YA, Huang CJ et al (2015) Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci Rep 5:16522. https://doi.org/10.1038/srep16522
Moon YJ, Yun CY, Choi H et al (2018) Osterix regulates corticalization for longitudinal bone growth via integrin β3 expression. Exp Mol Med 50(7):1–11. https://doi.org/10.1038/s12276-018-0119-9
Stich T, Alagboso F, Křenek T et al (2021) Implant-bone-interface: reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng Transl Med 7(1):e10239. https://doi.org/10.1002/btm2.10239
Su T, Zheng A, Cao L et al (2022) Adhesion-enhancing coating embedded with osteogenesis-promoting PDA/HA nanoparticles for peri-implant soft tissue sealing and osseointegration. Bio-Des Manuf 5(2):233–248. https://doi.org/10.1007/s42242-022-00184-5
Farzin A, Hassan S, Teixeira LSM et al (2021) Self-oxygenation of tissues orchestrates full-thickness vascularization of living implants. Adv Funct Mater 31(42):2100850. https://doi.org/10.1002/adfm.202100850
Kocherova I, Bryja A, Mozdziak P et al (2019) Human umbilical vein endothelial cells (HUVECs) co-culture with osteogenic cells: from molecular communication to engineering prevascularised bone grafts. J Clin Med 8(10):1602. https://doi.org/10.3390/jcm8101602
Dashnyam K, Buitrago JO, Bold T et al (2019) Angiogenesis-promoted bone repair with silicate-shelled hydrogel fiber scaffolds. Biomater Sci 7(12):5221–5231. https://doi.org/10.1039/c9bm01103j
Trujillo S, Gonzalez-Garcia C, Rico P et al (2020) Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 252:120104. https://doi.org/10.1016/j.biomaterials
Li J, Liu X, Crook JM et al (2020) 3D printing of cytocompatible graphene/alginate scaffolds for mimetic tissue constructs. Front Bioeng Biotechnol 8:824. https://doi.org/10.3389/fbioe.2020.00824
Vande Walle L, Lamkanfi M (2011) Inflammasomes: caspase-1-activating platforms with critical roles in host defense. Front Microbiol 2:3. https://doi.org/10.3389/fmicb.2011.00003
Tan F, Al-Rubeai M (2021) A multifunctional dexamethasone-delivery implant fabricated using atmospheric plasma and its effects on apoptosis, osteogenesis and inflammation. Drug Deliv Transl Res 11(1):86–102. https://doi.org/10.1007/s13346-019-00700-8
Lin TH, Pajarinen J, Lu L et al (2017) NF-κB as a therapeutic target in inflammatory-associated bone diseases. Adv Protein Chem Struct Biol 107:117–154. https://doi.org/10.1016/bs.apcsb.2016.11.002
Yu C, Zhang C, Kuang Z et al (2021) The role of NLRP3 inflammasome activities in bone diseases and vascular calcification. Inflammation 44(2):434–449. https://doi.org/10.1007/s10753-020-01357-z
Rocha FRG, Delitto AE, de Souza JAC et al (2020) Relevance of Caspase-1 and NLRP3 inflammasome on inflammatory bone resorption in a murine model of periodontitis. Sci Rep 10(1):7823. https://doi.org/10.1038/s41598-020-64685-y
Ma XY, Cui D, Wang Z et al (2022) Silk fibroin/hydroxyapatite coating improved osseointegration of porous titanium implants under diabetic conditions via activation of the PI3K/Akt signaling pathway. ACS Biomater Sci Eng 8(7):2908–2919. https://doi.org/10.1021/acsbiomaterials.2c00023
Zhao C, Qiu P, Li M et al (2021) The spatial form periosteal-bone complex promotes bone regeneration by coordinating macrophage polarization and osteogenic-angiogenic events. Mater Today Bio 12:100142. https://doi.org/10.1016/j.mtbio.2021.100142
Tan L, Fu J, Feng F et al (2020) Engineered probiotics biofilm enhances osseointegration via immunoregulation and anti-infection. Sci Adv 6(46):eaba5723. https://doi.org/10.1126/sciadv.aba5723
He M, Gao X, Fan Y et al (2021) Tannic acid/Mg2+-based versatile coating to manipulate the osteoimmunomodulation of implants. J Mater Chem B 9(4):1096–1106. https://doi.org/10.1039/d0tb01577f
Peng F, Qiu L, Yao M et al (2021) A lithium-doped surface inspires immunomodulatory functions for enhanced osteointegration through PI3K/AKT signaling axis regulation. Biomater Sci 9(24):8202–8220. https://doi.org/10.1039/d1bm01075a
Chen M, Hu J, Zhang E et al (2021) The osteoimmunomodulatory effect of nanostructured TiFx/TiOx coating on osteogenesis induction. Biomed Mater 16(4):045041. https://doi.org/10.1088/1748-605X/ac0863
Ding T, Kang W, Li J et al (2021) An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J Nanobiotechnol 19(1):247. https://doi.org/10.1186/s12951-021-00992-4
Ma Z, He HT, Deng CX et al (2022) 3D bioprinting of proangiogenic constructs with induced immunomodulatory microenvironments through a dual cross-linking procedure using laponite incorporated bioink. Composites Part B Eng 229:109399. https://doi.org/10.1016/j.compositesb.2021.109399
Shi J, Sun J, Zhang W et al (2016) Demineralized bone matrix scaffolds modified by CBD-SDF-1α promote bone regeneration via recruiting endogenous stem cells. ACS Appl Mater Interf 8(41):27511–27522. https://doi.org/10.1021/acsami.6b08685
Steen EH, Wang X, Balaji S et al (2020) The role of the anti-inflammatory cytokine Interleukin-10 in tissue fibrosis. Adv Wound Care 9(4):184–198. https://doi.org/10.1089/wound.2019.1032
Li K, Lu X, Liu S et al (2021) Boron-incorporated micro/nano-topographical calcium silicate coating dictates osteo/angio-genesis and inflammatory response toward enhanced osseointegration. Biol Trace Elem Res 199(10):3801–3816. https://doi.org/10.1007/s12011-020-02517-w
Wang L, Luo Q, Zhang X et al (2020) Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts. Bioact Mater 6(1):64–74. https://doi.org/10.1016/j.bioactmat.2020.07.012
Palkowitz AL, Tuna T, Bishti S et al (2021) Biofunctionalization of dental abutment surfaces by crosslinked ECM proteins strongly enhances adhesion and proliferation of gingival fibroblasts. Adv Healthc Mater 10(10):e2100132. https://doi.org/10.1002/adhm.202100132
Chen W, Xu K, Tao B et al (2018) Multilayered coating of titanium implants promotes coupled osteogenesis and angiogenesis in vitro and in vivo. Acta Biomater 74:489–504. https://doi.org/10.1016/j.actbio.2018.04.043
Zheng W, Chen C, Zhang X et al (2021) Layer-by-layer coating of carboxymethyl chitosan gelatin-alginate on cotton gauze for hemostasis and wound healing. Surf Coat Technol 406:126644. https://doi.org/10.1016/j.surfcoat.2020.126644
Fisher LE, Kämmerling L, Alexander MR et al (2022) Immune-instructive materials as new tools for immunotherapy. Curr Opin Biotechnol 74:194–203. https://doi.org/10.1016/j.copbio.2021.11.005
Rondanelli M, Faliva MA, Peroni G et al (2020) Pivotal role of boron supplementation on bone health: a narrative review. J Trace Elem Med Biol 62:126577. https://doi.org/10.1016/j.jtemb.2020.126577
Acknowledgements
Authors would like to thank Center of Excellence in Biomaterials and Tissue Engineering (BIOMATEN) for the support provided. Authors also acknowledge financial support provided by National Boron Institute (BOREN, Grant No: 2018-31-07-25-001).
Author information
Authors and Affiliations
Contributions
Conceptualization: AEP, KA, ZE and AT; methodology: AEP, TD and MVY; data analysis: AEP and TD; writing—original draft preparation: AEP; writing—review and editing: DK, ZE and AT; ZE provided sources; KA, ZE and AT performed supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal procedures in our study were approved by the Institutional Animal Care and Use Committee of Afyon Kocatepe University (Date: 18.09.2018, Decision No.: 49533702/147).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pazarçeviren, A.E., Evis, Z., Dikmen, T. et al. Alginate/gelatin/boron-doped hydroxyapatite-coated Ti implants: in vitro and in vivo evaluation of osseointegration. Bio-des. Manuf. 6, 217–242 (2023). https://doi.org/10.1007/s42242-022-00218-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-022-00218-y