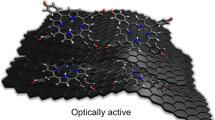
Abstract
Vectored non-covalent interactions—mainly hydrogen bonding and aromatic interactions—extensively contribute to (bio)-organic self-assembling processes and significantly impact the physicochemical properties of the associated superstructures. However, vectored non-covalent interaction-driven assembly occurs mainly along one-dimensional (1D) or three-dimensional (3D) directions, and a two-dimensional (2D) orientation, especially that of multilayered, graphene-like assembly, has been reported less. In this present research, by introducing amino, hydroxyl, and phenyl moieties to the triazine skeleton, supramolecular layered assembly is achieved by vectored non-covalent interactions. The planar hydrogen bonding network results in high stability, with a thermal sustainability of up to about 330 °C and a Young’s modulus of up to about 40 GPa. Upon introducing wrinkles by biased hydrogen bonding or aromatic interactions to disturb the planar organization, the stability attenuates. However, the intertwined aromatic interactions prompt a red edge excitation shift effect inside the assemblies, inducing broad-spectrum fluorescence covering nearly the entire visible light region (400–650 nm). We show that bionic, superhydrophobic, pillar-like arrays with contact angles of up to about 170° can be engineered by aromatic interactions using a physical vapor deposition approach, which cannot be realized through hydrogen bonding. Our findings show the feasibility of 2D assembly with engineerable properties by modulating vectored non-covalent interactions.
Graphic abstract









Similar content being viewed by others
References
Aida T, Meijer EW, Stupp SI (2018) Functional supramolecular polymers. Science 335:813–817. https://doi.org/10.1126/science.1205962
Whitesides GM, Grzybowski B (2002) Self-assembly at all scales. Science 295:2418–2421. https://doi.org/10.1126/science.1070821
Yan X, Zhu P, Li J (2010) Self-assembly and application of diphenylalanine-based nanostructures. Chem Soc Rev 39:1877–1890. https://doi.org/10.1039/b915765b
Yuan C, Ji W, Xing R et al (2019) Hierarchically oriented organization in supramolecular peptide crystals. Nat Rev Chem 3:567–588. https://doi.org/10.1038/s41570-019-0129-8
Tao K, Makam P, Aizen R et al (2017) Self-assembling peptide semiconductors. Science. https://doi.org/10.1126/science.aam9756.
Fleming S, Ulijn RV (2014) Design of nanostructures based on aromatic peptide amphiphiles. Chem Soc Rev 43:8150–8177. https://doi.org/10.1039/c4cs00247d
Hong Y, Lam JWY, Tang BZ (2011) Aggregation-induced emission. Chem Soc Rev 40:5361–5388. https://doi.org/10.1039/C1CS15113D
Zhao X, Pan F, Xu H et al (2010) Molecular self-assembly and applications of designer peptide amphiphiles. Chem Soc Rev 39:3480–3498. https://doi.org/10.1039/b915923c
Ke PC, Sani MA, Ding F et al (2017) Implications of peptide assemblies in amyloid diseases. Chem Soc Rev 46:6492–6531. https://doi.org/10.1039/c7cs00372b
Zhang S (2003) Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol 21:1171–1178. https://doi.org/10.1038/nbt874
Sun B, Tao K, Jia Y et al (2019) Photoactive properties of supramolecular assembled short peptides. Chem Soc Rev 48:4387–4400. https://doi.org/10.1039/c9cs00085b
Tao K, Levin A, Adler-Abramovich L et al (2016) Fmoc-modified amino acids and short peptides: simple bio-inspired building blocks for the fabrication of functional materials. Chem Soc Rev 45:3935–3953. https://doi.org/10.1039/c5cs00889a
Wei G, Xi W, Nussinov R et al (2016) Protein ensembles: how does nature harness thermodynamic fluctuations for life? The diverse functional roles of conformational ensembles in the cell. Chem Rev 116:6516–6551. https://doi.org/10.1021/acs.chemrev.5b00562
Knowles TPJ, Buehler MJ (2011) Nanomechanics of functional and pathological amyloid materials. Nat Nanotechnol 6:469–479. https://doi.org/10.1038/nnano.2011.102
Mei J, Leung NLC, Kwok RTK et al (2015) Aggregation-induced emission: together we shine, united we soar! Chem Rev 115:11718–11940. https://doi.org/10.1021/acs.chemrev.5b00263
Lim X (2016) The nanolight revolution is coming. Nature 531:26–28. https://doi.org/10.1038/531026a
Luo J, Xie Z, Lam JWY et al (2001) Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem Commun 24:1740–1741. https://doi.org/10.1039/b105159h
Fan Z, Sun L, Huang Y et al (2016) Bioinspired fluorescent dipeptide nanoparticles for targeted cancer cell imaging and real-time monitoring of drug release. Nat Nanotechnol 11:388–394. https://doi.org/10.1038/nnano.2015.312
Tao K, Fan Z, Sun L et al (2018) Quantum confined peptide assemblies with tunable visible to near-infrared spectral range. Nat Commun 9:3217. https://doi.org/10.1038/s41467-018-05568-9
Gao Y, Zhao F, Wang Q et al (2010) Small peptide nanofibers as the matrices of molecular hydrogels for mimicking enzymes and enhancing the activity of enzymes. Chem Soc Rev 39:3425–3433. https://doi.org/10.1039/b919450a
Günes S, Neugebauer H, Sariciftci NS (2007) Conjugated polymer-based organic solar cells. Chem Rev 107:1324–1338. https://doi.org/10.1021/cr050149z
Mishra A, Bäuerle P (2012) Small molecule organic semiconductors on the move: promises for future solar energy technology. Angew Chem Int Edit 51:2020–2067. https://doi.org/10.1002/anie.201102326
Hauser CAE, Zhang S (2010) Peptides as biological semiconductors. Nature 468:516–517. https://doi.org/10.1038/468516a
Marvin CW, Grim HM, Miller NC et al (2017) Interplay among sequence, folding propensity, and bio-piezoelectric response in short peptides and peptoids. J Phys Chem B 121:10269–10275. https://doi.org/10.1021/acs.jpcb.7b10085
Liu P, Pan C, Wang ZL (2018) Two-dimensional nanomaterials for novel piezotronics and piezophototronics. Mater Today Nano 4:17–31. https://doi.org/10.1016/j.mtnano.2018.11.006
Zelenovskii PS, Romanyuk K, Liberato MS et al (2021) 2D layered dipeptide crystals for piezoelectric applications. Adv Funct Mater 31:2102524. https://doi.org/10.1002/adfm.202102524
Argaman N, Makov G (2000) Density functional theory: an introduction. Am J Phys 68:69–79. https://doi.org/10.1119/1.19375
Hafner J (2007) Materials simulations using VASP—a quantum perspective to materials science. Comput Phys Commun 177:6–13. https://doi.org/10.1016/j.cpc.2007.02.045
Perdew JP, Chevary JA, Vosko SH et al (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671–6687. https://doi.org/10.1103/physrevb.46.6671
Grimme S, Antony J, Ehrlich S et al (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775. https://doi.org/10.1103/PhysRevB.59.1758
Wu X, Vanderbilt D, Hamann D (2005) Systematic treatment of displacements, strains, and electric fields in density-functional perturbation theory. Phys Rev B 72:035105. https://doi.org/10.1103/PhysRevB.72.035105
Chung DH, Buessem WR (1967) The Voigt-Reuss-Hill approximation and elastic moduli of polycrystalline MgO, CaF2, β-ZnS, ZnSe, and CdTe. J Appl Phys 38:2535–2540. https://doi.org/10.1063/1.1709944
Zuo L, Humbert M, Esling C (1992) Elastic properties of polycrystals in the Voigt-Reuss-Hill approximation. J Appl Cryst 25:751–755. https://doi.org/10.1107/S0021889892004874
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Cryst 44:1272–1276. https://doi.org/10.1107/S0021889811038970
Tao K, Xue B, Li Q et al (2019) Stable and optoelectronic dipeptide assemblies for power harvesting. Mater Today 30:10–16. https://doi.org/10.1016/j.mattod.2019.04.002
Tao K, Hu W, Xue B et al (2019) Bioinspired stable and photoluminescent assemblies for power generation. Adv Mater 31:1807481. https://doi.org/10.1002/adma.201807481
Tao K, O’Donnell J, Yuan H et al (2020) Accelerated charge transfer in water-layered peptide assemblies. Energy Environ Sci 13:96–101. https://doi.org/10.1039/c9ee02875g
Bystrov VS, Coutinho J, Zhulyabina OA et al (2021) Modeling and physical properties of diphenylalanine peptide nanotubes containing water molecules. Ferroelectrics 574:78–91. https://doi.org/10.1080/00150193.2021.1888051
Berger O, Adler-Abramovich L, Levy-Sakin M et al (2015) Light-emitting self-assembled peptide nucleic acids exhibit both stacking interactions and Watson-Crick base pairing. Nat Nanotechnol 10:353–360. https://doi.org/10.1038/nnano.2015.27
Hod O (2012) Graphite and hexagonal boron-nitride have the same interlayer distance. Why? J Chem Theory Comput 8:1360–1369. https://doi.org/10.1021/ct200880m
Tao K, Tang Y, Rencus-Lazar S et al (2020) Bioinspired supramolecular packing enables high thermo-sustainability. Angew Chem 132:19199–19203. https://doi.org/10.1002/anie.202008702
Azuri I, Adler-Abramovich L, Gazit E et al (2014) Why are diphenylalanine-based peptide nanostructures so rigid? Insights from first principles calculations. J Am Chem Soc 136:963–969. https://doi.org/10.1021/ja408713x
Knowles TP, Fitzpatrick AW, Meehan S et al (2007) Role of intermolecular forces in defining material properties of protein nanofibrils. Science 318:1900–1903. https://doi.org/10.1126/science.1150057
Ranganathan S, Maji SK, Padinhateeri R (2016) Defining a physical basis for diversity in protein self-assemblies using a minimal model. J Am Chem Soc 138:13911–13922. https://doi.org/10.1021/jacs.6b06433
Papageorgiou DG, Kinloch IA, Young RJ (2017) Mechanical properties of graphene and graphene-based nanocomposites. Prog Mater Sci 90:75–127. https://doi.org/10.1016/j.pmatsci.2017.07.004
Frank IW, Tanenbaum DM, van der Zande AM et al (2007) Mechanical properties of suspended graphene sheets. J Vac Sc Technol B 25:2558–2561. https://doi.org/10.1116/1.2789446
Mei J, Hong Y, Lam JWY et al (2014) Aggregation-induced emission: the whole is more brilliant than the parts. Adv Mater 26:5429–5479. https://doi.org/10.1002/adma.201401356
Chattopadhyay A, Haldar S (2014) Dynamic insight into protein structure utilizing red edge excitation shift. Acc Chem Res 47:12–19. https://doi.org/10.1021/ar400006z
Rosenman G, Beker P, Koren I et al (2011) Bioinspired peptide nanotubes: deposition technology, basic physics and nanotechnology applications. J Pept Sci 17:75–87. https://doi.org/10.1002/psc.1326
Kasotakis E, Mossou E, Adler-Abramovich L et al (2009) Design of metal-binding sites onto self-assembled peptide fibrils. Biopolymers 92:164–172. https://doi.org/10.1002/bip.21163
Bank-Srour B, Becker P, Krasovitsky L et al (2013) Physical vapor deposition of peptide nanostructures. Polym J 45:494–503. https://doi.org/10.1038/pj.2013.19
Sun M, Luo C, Xu L et al (2005) Artificial lotus leaf by nanocasting. Langmuir 21:8978–8981. https://doi.org/10.1021/la050316q
Lipomi DJ, Vosgueritchian M, Tee BCK et al (2011) Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat Nanotechnol 6:788–792. https://doi.org/10.1038/nnano.2011.184
Wu P, Wang J, Jiang L (2020) Bio-inspired photonic crystal patterns. Mater Horiz 7:338–365. https://doi.org/10.1039/C9MH01389J
Gu L, Shi H, Bian L et al (2019) Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat Photonics 13:406–411. https://doi.org/10.1038/s41566-019-0408-4
Acknowledgements
This work was supported by the Fund for Creative Research Groups of National Natural Science Foundation of China (No. 51821093), the National Natural Science Foundation of China (Nos. 52175551, 52075484) (KT and DM), the National Key Research and Development Program (SQ2021YFE010405) (KT) and Science Foundation Ireland (SFI) through awards Nos. 15/CDA/3491 and 12/RC/2275_P2 (DT), and computing resources at the SFI/Higher Education Authority Irish Center for High-End Computing (ICHEC) (SG and DT). The authors thank Dr. Sigal Rencus-Lazar for language editing and the members of all the laboratories for helpful discussions.
Author information
Authors and Affiliations
Contributions
All authors contributed to this manuscript and gave permission for the final version of the publication. BX, DT, and KT conceived and designed the work; JZ, HW, YW, and DM conducted the crystal growth and further characterizations; BX, YC, and WW conducted the Young’s model measurement; JZ, HW, and EG performed the TGA experiments; JZ, HW, YW, DM, and EG fabricated the PVD system and the coating film; SG, SAMT, and DT carried out theoretical calculations; JZ and SG coordinated all the work, analyzed the results, wrote and edited the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Guerin, S., Wu, H. et al. Modulating vectored non-covalent interactions for layered assembly with engineerable properties. Bio-des. Manuf. 5, 529–539 (2022). https://doi.org/10.1007/s42242-022-00186-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-022-00186-3