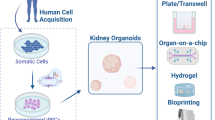
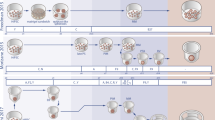
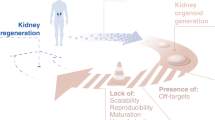
Abstract
Despite a continuing increase in the number of patients suffering from chronic kidney disease, currently available treatments for these patients, such as dialysis and kidney transplantation, are imperfect. The kidney is also a critical target organ vulnerable to the toxicity of various new drugs, and the lack of a reliable in vitro culture model imposes a severe limitation on drug discovery. Although the development of induced pluripotent stem cells (iPSCs) revolutionized strategies in biomedical fields, the complexity of the kidney imposed additional challenge to the application of this technology in kidney regeneration. Nonetheless, the recent advancement in our understanding on the developmental origin of kidney progenitor cells and the mechanisms of their reciprocal induction and self-organization has boosted research in kidney regeneration. Research since then has demonstrated that kidney organoids derived from iPSCs can serve as a useful model for drug discovery and toxicity screening, as well as for disease modeling, especially in combination with gene editing techniques. Moreover, attempts at kidney organoid implantation in animals have suggested their potential as an alternative source of kidney transplantation. In this review, we summarize recent progress on the generation of kidney organoids, as well as the obstacles that remain.




Similar content being viewed by others
References
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. https://doi.org/10.1016/j.cell.2007.11.019
Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA, Nakauchi H (2013) Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci USA 110:4557–4562. https://doi.org/10.1073/pnas.1222902110
Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H (2012) Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol 180:2417–2426. https://doi.org/10.1016/j.ajpath.2012.03.007
Song JJ, Guyette J, Gilpin S, Gonzalez G, Vacanti JP, Ott HC (2013) Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19:646–651. https://doi.org/10.1038/nm.3154
Yokote S, Matsunari H, Iwai S, Yamanaka S, Uchikura A, Fujimoto E, Matsumoto K, Nagashima H, Kobayashi E, Yokoo T (2015) Urine excretion strategy for stem cell-generated embryonic kidneys. Proc Natl Acad Sci USA 112:12980–12985. https://doi.org/10.1073/pnas.1507803112
Oxburgh L, Carroll TJ, Cleaver O, Gossett DR, Hoshizaki DK, Hubbell JA, Humphreys BD, Jain S, Jensen J, Kaplan DL, Kesselman C, Ketchum CJ, Little MH, McMahon AP, Shankland SJ, Spence JR, Valerius MT, Wertheim JA, Wessely O, Zheng Y, Drummond IA (2017) (Re)Building a Kidney. J Am Soc Nephrol 28:1370–1378. https://doi.org/10.1681/ASN.2016101077
Unbekandt M, Davies JA (2010) Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int 77:407–416. https://doi.org/10.1038/ki.2009.482
Murakami Y, Naganuma H, Tanigawa S, Fujimori T, Eto M, Nishinakamura R (2019) Reconstitution of the embryonic kidney identifies a donor cell contribution to the renal vasculature upon transplantation. Sci Rep 9:1172. https://doi.org/10.1038/s41598-018-37793-z
Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, de Sousa Chuva, Lopes SM, Little MH (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526:564–568. https://doi.org/10.1038/nature15695
Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV (2015) Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6:1–13. https://doi.org/10.1038/ncomms9715
Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K, Bennetts B, Crawford J, Trnka P, Oshlack A, Patel C, Mallett A, Simons C, Little MH (2018) Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet 102:816–831. https://doi.org/10.1016/j.ajhg.2018.03.014
Tanigawa S, Islam M, Sharmin S, Naganuma H, Yoshimura Y, Haque F, Era T, Nakazato H, Nakanishi K, Sakuma T, Yamamoto T, Kurihara H, Taguchi A, Nishinakamura R (2018) Organoids from nephrotic disease-derived iPSCs identify impaired NEPHRIN localization and slit diaphragm formation in kidney podocytes. Stem Cell Rep 11:727–740. https://doi.org/10.1016/j.stemcr.2018.08.003
Hoenig MP, Zeidel ML (2014) Homeostasis, the milieu intérieur, and the wisdom of the nephron. Clin J Am Soc Nephrol 9:1272–1281. https://doi.org/10.2215/CJN.08860813
Little MH, McMahon AP (2012) Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 4:a008300. https://doi.org/10.1101/cshperspect.a008300
Costantini F, Kopan R (2010) Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18:698–712. https://doi.org/10.1016/j.devcel.2010.04.008
Rowan CJ, Sheybani-Deloui S, Rosenblum ND (2017) Origin and function of the renal stroma in health and disease. Results Probl Cell Differ 60:205–229. https://doi.org/10.1007/978-3-319-51436-9_8
Fanni D, Gerosa C, Vinci L, Ambu R, Dessì A, Eyken PV, Fanos V, Faa G (2016) Interstitial stromal progenitors during kidney development: here, there and everywhere. J Maternal-Fetal Neonatal Med 29:3815–3820. https://doi.org/10.3109/14767058.2016.1147553
Nagalakshmi VK, Yu J (2015) The ureteric bud epithelium: morphogenesis and roles in metanephric kidney patterning. Mol Reprod Dev 82:151–166. https://doi.org/10.1002/mrd.22462
Dressler GR (2009) Advances in early kidney specification, development and patterning. Development 136:3863–3874. https://doi.org/10.1242/dev.034876
Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14:53–67. https://doi.org/10.1016/j.stem.2013.11.010
Araoka T, Mae S, Kurose Y, Uesugi M, Ohta A, Yamanaka S, Osafune K (2014) Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PLoS ONE 9:e84881. https://doi.org/10.1371/journal.pone.0084881
Mae S-I, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, Arai S, Sato-Otubo A, Toyoda T, Takahashi K, Nakayama N, Cowan CA, Aoi T, Ogawa S, McMahon AP, Yamanaka S, Osafune K (2013) Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun 4:1367. https://doi.org/10.1038/ncomms2378
Taguchi A, Nishinakamura R (2017) Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21:730–746.e6. https://doi.org/10.1016/j.stem.2017.10.011
Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33:1193–1200. https://doi.org/10.1038/nbt.3392
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16:118–126. https://doi.org/10.1038/ncb2894
Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih J-H, Wolvetang EJ, McMahon AP, Holm TM, Davidson AJ (2018) A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep 11:470–484. https://doi.org/10.1016/j.stemcr.2018.06.018
Kandasamy K, Chuah JKC, Su R, Huang P, Eng KG, Xiong S, Li Y, Chia CS, Loo L-H, Zink D (2015) Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Sci Rep 5:12337. https://doi.org/10.1038/srep12337
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818. https://doi.org/10.1001/jama.294.7.813
Kim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, Cruz NM, Liu Y, Churchill AJ, Wang Y, Fu H, Pippin JW, Lin LY, Shankland SJ, Vogl AW, McNagny KM, Freedman BS (2017) Gene-edited human kidney organoids reveal mechanisms of disease in podocyte development. Stem Cells 35:2366–2378. https://doi.org/10.1002/stem.2707
Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu M-Z, Dubova I, Esteban CR, Montserrat N, Campistol JM, Izpisua Belmonte JC (2013) Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol 15:1507–1515. https://doi.org/10.1038/ncb2872
Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD (2018) Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23:869–881.e8. https://doi.org/10.1016/j.stem.2018.10.010
Combes AN, Zappia L, Er PX, Oshlack A, Little MH (2019) Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med 11:3. https://doi.org/10.1186/s13073-019-0615-0
Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R (2019) Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16:255–262. https://doi.org/10.1038/s41592-019-0325-y
Rogers SA, Lowell JA, Hammerman NA, Hammerman MR (1998) Transplantation of developing metanephroi into adult rats. Kidney Int 54:27–37. https://doi.org/10.1046/j.1523-1755.1998.00971.x
Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, Rechavi G, Friedman N, Kaminski N, Passwell JH, Reisner Y (2003) Human and porcine early kidney precursors as a new source for transplantation. Nat Med 9:53–60. https://doi.org/10.1038/nm812
Kim S-S, Gwak S-J, Han J, Park HJ, Park MH, Song KW, Cho SW, Rhee YH, Chung HM, Kim B-S (2007) Kidney tissue reconstruction by fetal kidney cell transplantation: effect of gestation stage of fetal kidney cells. Stem Cells 25:1393–1401. https://doi.org/10.1634/stemcells.2006-0183
Ji RP, Phoon CKL, Aristizábal O, McGrath KE, Palis J, Turnbull DH (2003) Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ Res 92:133–135. https://doi.org/10.1161/01.res.0000056532.18710.c0
Tufro A, Norwood VF, Carey RM, Gomez RA (1999) Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. JASN 10:2125–2134
Munro DAD, Hohenstein P, Davies JA (2017) Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci Rep 7:3273. https://doi.org/10.1038/s41598-017-03808-4
Daniel E, Azizoglu DB, Ryan AR, Walji TA, Chaney CP, Sutton GI, Carroll TJ, Marciano DK, Cleaver O (2018) Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis 21:617–634. https://doi.org/10.1007/s10456-018-9612-y
Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, Mukoyama M, Yamamoto T, Kurihara H, Nishinakamura R (2016) Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol 27:1778–1791. https://doi.org/10.1681/ASN.2015010096
Bantounas I, Ranjzad P, Tengku F, Silajdžić E, Forster D, Asselin M-C, Lewis P, Lennon R, Plagge A, Wang Q, Woolf AS, Kimber SJ (2018) Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Rep 10:766–779. https://doi.org/10.1016/j.stemcr.2018.01.008
Hyink DP, Tucker DC, St John PL, Leardkamolkarn V, Accavitti MA, Abrass CK, Abrahamson DR (1996) Endogenous origin of glomerular endothelial and mesangial cells in grafts of embryonic kidneys. Am J Physiol 270:F886–F899. https://doi.org/10.1152/ajprenal.1996.270.5.F886
Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, Conti S, Unbekandt M, Davies JA, Morigi M, Benigni A, Remuzzi G (2012) In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol 23:1857–1868. https://doi.org/10.1681/ASN.2012050505
Halt KJ, Pärssinen HE, Junttila SM, Saarela U, Sims-Lucas S, Koivunen P, Myllyharju J, Quaggin S, Skovorodkin IN, Vainio SJ (2016) CD146(+) cells are essential for kidney vasculature development. Kidney Int 90:311–324. https://doi.org/10.1016/j.kint.2016.02.021
Rosines E, Johkura K, Zhang X, Schmidt HJ, DeCambre M, Bush KT, Nigam SK (2010) Constructing kidney-like tissues from cells based on programs for organ development: toward a method of in vitro tissue engineering of the kidney. Tissue Eng Part A 16:2441–2455. https://doi.org/10.1089/ten.tea.2009.0548
Ganeva V, Unbekandt M, Davies JA (2011) An improved kidney dissociation and reaggregation culture system results in nephrons arranged organotypically around a single collecting duct system. Organogenesis 7:83–87. https://doi.org/10.4161/org.7.2.14881
Mills CG, Lawrence ML, Munro DAD, Elhendawi M, Mullins JJ, Davies JA (2017) Asymmetric BMP4 signalling improves the realism of kidney organoids. Sci Rep 7:1–8. https://doi.org/10.1038/s41598-017-14809-8
Hauser PV, Nishikawa M, Kimura H, Fujii T, Yanagawa N (2016) Controlled tubulogenesis from dispersed ureteric bud-derived cells using a micropatterned gel. J Tissue Eng Regen Med 10:762–771. https://doi.org/10.1002/term.1871
Takasato M, Little MH (2015) The origin of the mammalian kidney: implications for recreating the kidney in vitro. Development 142:1937–1947. https://doi.org/10.1242/dev.104802
Nishikawa M, Kimura H, Yanagawa N, Hamon M, Hauser P, Zhao L, Jo OD, Yanagawa N (2018) An optimal serum-free defined condition for in vitro culture of kidney organoids. Biochem Biophys Res Commun 501:996–1002. https://doi.org/10.1016/j.bbrc.2018.05.098
Kumar SV, Er PX, Lawlor KT, Motazedian A, Scurr M, Ghobrial I, Combes AN, Zappia L, Oshlack A, Stanley EG, Little MH (2019) Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development. https://doi.org/10.1242/dev.172361
Tanigawa S, Taguchi A, Sharma N, Perantoni AO, Nishinakamura R (2016) Selective in vitro propagation of nephron progenitors derived from embryos and pluripotent stem cells. Cell Rep 15:801–813. https://doi.org/10.1016/j.celrep.2016.03.076
Li Z, Araoka T, Wu J, Liao H-K, Li M, Lazo M, Zhou B, Sui Y, Wu M-Z, Tamura I, Xia Y, Beyret E, Matsusaka T, Pastan I, Rodriguez Esteban C, Guillen I, Guillen P, Campistol JM, Izpisua Belmonte JC (2016) 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell 19:516–529. https://doi.org/10.1016/j.stem.2016.07.016
Brown AC, Muthukrishnan SD, Oxburgh L (2015) A synthetic niche for nephron progenitor cells. Dev Cell 34:229–241. https://doi.org/10.1016/j.devcel.2015.06.021
Yuri S, Nishikawa M, Yanagawa N, Jo OD, Yanagawa N (2015) Maintenance of mouse nephron progenitor cells in aggregates with gamma-secretase inhibitor. PLoS ONE 10:e0129242. https://doi.org/10.1371/journal.pone.0129242
Yuri S, Nishikawa M, Yanagawa N, Jo OD, Yanagawa N (2017) In Vitro propagation and branching morphogenesis from single ureteric bud cells. Stem Cell Reports 8:401–416. https://doi.org/10.1016/j.stemcr.2016.12.011
Lemos DR, McMurdo M, Karaca G, Wilflingseder J, Leaf IA, Gupta N, Miyoshi T, Susa K, Johnson BG, Soliman K, Wang G, Morizane R, Bonventre JV, Duffield JS (2018) Interleukin-1β activates a MYC-dependent metabolic switch in kidney stromal cells necessary for progressive tubulointerstitial fibrosis. JASN 29:1690–1705. https://doi.org/10.1681/ASN.2017121283
O’Brien LL, Guo Q, Lee Y, Tran T, Benazet J-D, Whitney PH, Valouev A, McMahon AP (2016) Differential regulation of mouse and human nephron progenitors by the Six family of transcriptional regulators. Development 143:595–608. https://doi.org/10.1242/dev.127175
Lindström NO, Guo J, Kim AD, Tran T, Guo Q, De Sena Brandine G, Ransick A, Parvez RK, Thornton ME, Baskin L, Grubbs B, McMahon JA, Smith AD, McMahon AP (2018) Conserved and divergent features of mesenchymal progenitor cell types within the cortical nephrogenic niche of the human and mouse kidney. J Am Soc Nephrol 29:806–824. https://doi.org/10.1681/ASN.2017080890
Acknowledgements
Funding was provided by Japan Society for the Promotion of Science (Grant No. Grant-in-Aid for Research Activity Start-up (19K23600) and the Chau-Li Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Nishikawa, M., Sakai, Y. & Yanagawa, N. Design and strategy for manufacturing kidney organoids. Bio-des. Manuf. 3, 7–14 (2020). https://doi.org/10.1007/s42242-020-00060-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-020-00060-0