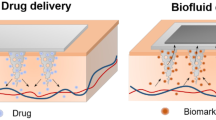
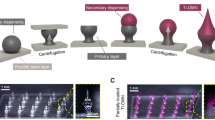
Abstract
The advancement in the materials manufacturing at micrometer and nanometer scales has already enabled numerous applications in electronics, optics, chemistry, biology and medicine. Biomedical devices carrying micro-/nanostructures are currently being widely used in drug delivery, drug release, biosensing and therapy. New clinical methods for disease diagnosis and treatments are being developed enabled by nanotechnology. One-dimensional (1D) structures are playing an important role in the direct drug delivery both in vivo and ex vivo among various micro-/nanostructures. Here, in this paper, we reviewed recent progresses made on next-generation intradermal and intracellular delivery strategies and applications with focus on 1D microstructure-based approaches.



Similar content being viewed by others
References
Kim K, Xu X, Guo J, Fan DL (2014) Ultrahigh-speed rotating nanoelectromechanical system devices assembled from nanoscale building blocks. Nat Commun 5:3632
Xu X, Kim K, Fan D (2015) Tunable release of multiplex biochemicals by plasmonically active rotary nanomotors. Angew Chem Int Ed 54(8):2525–2529
Liao W-S, Cheunkar S, Cao HH, Bednar HR, Weiss PS, Andrews AM (2012) Subtractive patterning via chemical lift-off lithography. Science 337(6101):1517–1521
Xu X, Yang Q, Cheung KM, Zhao C, Wattanatorn N, Belling JN, Abendroth JM, Slaughter LS, Mirkin CA, Andrews AM, Weiss PS (2017) Polymer-pen chemical lift-off lithography. Nano Lett 17:3302–3311
Xu X, Yang Q, Wattanatorn N, Zhao C, Chiang N, Jonas SJ, Weiss PS (2017) Multiple-patterning nanosphere lithography for fabricating periodic three-dimensional hierarchical nanostructures. ACS Nano 11:10384–10391
Dai S, Chu Y, Liu D, Cao F, Wu X, Zhou J, Zhou B, Chen Y, Huang J (2018) Intrinsically ionic conductive cellulose nanopapers applied as all solid dielectrics for low voltage organic transistors. Nat Commun 9(1):2737
Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S, Balogh LP, Ballerini L, Bestetti A, Brendel C, Bosi S, Carril M, Chan WCW, Chen C, Chen X, Chen X, Cheng Z, Cui D, Du J, Dullin C, Escudero A, Feliu N, Gao M, George M, Gogotsi Y, Grünweller A, Gu Z, Halas NJ, Hampp N, Hartmann RK, Hersam MC, Hunziker P, Jian J, Jiang X, Jungebluth P, Kadhiresan P, Kataoka K, Khademhosseini A, Kopeček J, Kotov NA, Krug HF, Lee DS, Lehr C-M, Leong KW, Liang X-J, Ling Lim M, Liz-Marzán LM, Ma X, Macchiarini P, Meng H, Möhwald H, Mulvaney P, Nel AE, Nie S, Nordlander P, Okano T, Oliveira J, Park TH, Penner RM, Prato M, Puntes V, Rotello VM, Samarakoon A, Schaak RE, Shen Y, Sjöqvist S, Skirtach AG, Soliman MG, Stevens MM, Sung H-W, Tang BZ, Tietze R, Udugama BN, VanEpps JS, Weil T, Weiss PS, Willner I, Wu Y, Yang L, Yue Z, Zhang Q, Zhang Q, Zhang X-E, Zhao Y, Zhou X, Parak WJ (2017) Diverse applications of nanomedicine. ACS Nano 11(3):2313–2381
Choi J, Ghaffari R, Baker LB, Rogers JA (2018) Skin-interfaced systems for sweat collection and analytics. Sci Adv 4(2):3921
Jeong J-W, McCall JG, Shin G, Zhang Y, Al-Hasani R, Kim M, Li S, Sim JY, Jang K-I, Shi Y, Hong DY, Liu Y, Schmitz GP, Xia L, He Z, Gamble P, Ray WZ, Huang YY, Bruchas MR, Rogers JA (2015) Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162(3):662–674
Kim J, Gutruf P, Chiarelli AM, Heo SY, Cho K, Xie Z, Banks A, Han S, Jang K-I, Lee JW, Lee K-T, Feng X, Huang Y, Fabiani M, Gratton G, Paik U, Rogers JA (2017) Miniaturized battery-free wireless systems for wearable pulse oximetry. Adv Funct Mater 27(1):1604373
Ye Y, Yu J, Wen D, Kahkoska AR, Gu Z (2018) Polymeric microneedles for transdermal protein delivery. Adv Drug Deliv Rev 127:106–118
Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF (2016) In vitro and ex vivo strategies for intracellular delivery. Nature 538(7624):183–192
Wang J, Ye Y, Yu J, Kahkoska AR, Zhang X, Wang C, Sun W, Corder RD, Chen Z, Khan SA, Buse JB, Gu Z (2018) Core–shell microneedle gel for self-regulated insulin delivery. ACS Nano. https://doi.org/10.1021/acsnano.7b08152
Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, Kabbani S, Lai L, Vassilieva EV, Skountzou I, Compans RW, Mulligan MJ, Prausnitz MR, Beck A, Edupuganti S, Heeke S, Kelley C, Nesheim W (2017) The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 390(10095):649–658
Samant PP, Prausnitz MR (2018) Mechanisms of sampling interstitial fluid from skin using a microneedle patch. In: Proceedings of the National Academy of Sciences
Prausnitz MR (2015) Puncturing cells en masse. Nat Mater 14(5):470–471
Xie X, Xu AM, Leal-Ortiz S, Cao Y, Garner CC, Melosh NA (2013) Nanostraw-electroporation system for highly efficient intracellular delivery and transfection. ACS Nano 7(5):4351–4358
Lee JH, Jung YS, Kim GM, Bae JM (2018) A hyaluronic acid-based microneedle patch to treat psoriatic plaques: a pilot open trial. Brit J Dermatol 178(1):E24–E25
Zhang YQ, Yu JC, Wen D, Chen GJ, Gu Z (2018) The potential of a microneedle patch for reducing obesity. Expert Opin Drug Deliv 15(5):431–433
Kim JH, Shin JU, Kim SH, Noh JY, Kim HR, Lee J, Chu H, Jeong KY, Park KH, Kim JD, Kim HK, Jeong DH, Yong TS, Park JW, Lee KH (2018) Successful transdermal allergen delivery and allergen-specific immunotherapy using biodegradable microneedle patches. Biomaterials 150:38–48
Choi J-S, Zhu Y, Li H, Peyda P, Nguyen TT, Shen MY, Yang YM, Zhu J, Liu M, Lee MM, Sun S-S, Yang Y, Yu H-H, Chen K, Chuang GS, Tseng H-R (2017) Cross-linked fluorescent supramolecular nanoparticles as finite tattoo pigments with controllable intradermal retention times. ACS Nano 11(1):153–162
van der Maaden K, Jiskoot W, Bouwstra J (2012) Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release 161(2):645–655
Herwadkar A, Banga AK (2012) Peptide and protein transdermal drug delivery. Drug Discov Today Technol 9(2):e147–e154
Sivamani RK, Liepmann D, Maibach HI (2007) Microneedles and transdermal applications. Expert Opin Drug Deliv 4(1):19–25
Perez Cuevas MB, Kodani M, Choi Y, Joyce J, O’Connor SM, Kamili S, Prausnitz MR (2018) Hepatitis B vaccination using a dissolvable microneedle patch is immunogenic in mice and rhesus macaques. Bioeng Transl Med 3:186–196
Yim S, Jeon S, Kim JM, Baek KM, Lee GH, Kim H, Shin J, Jung YS (2018) Transferrable plasmonic au thin film containing sub-20 nm nanohole array constructed via high-resolution polymer self-assembly and nanotransfer printing. ACS Appl Mater Interfaces 10(3):2216–2223
Bennewitz NL, Babensee JE (2005) The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials 26(16):2991–2999
Boccafoschi F, Mosca C, Ramella M, Carmagnola I, Chiono V, Ciardelli G, Cannas M (2013) Biological evaluation of materials for cardiovascular application: the role of the short-term inflammatory response in endothelial regeneration. J Biomed Mater Res Part A 101(11):3131–3140
Jiang W, Tian Q, Vuong T, Shashaty M, Gopez C, Sanders T, Liu H (2017) Comparison study on four biodegradable polymer coatings for controlling magnesium degradation and human endothelial cell adhesion and spreading. ACS Biomater Sci Eng 3(6):936–950
Kim Y-C, Park J-H, Prausnitz MR (2012) Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 64(14):1547–1568
McAllister DV, Wang PM, Davis SP, Park J-H, Canatella PJ, Allen MG, Prausnitz MR (2003) Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci 100(24):13755–13760
Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol 26:1261
Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR (2001) Lack of pain associated with microfabricated microneedles. Anesth Analg 92(2):502–504
Yang JB, Chen ZP, Ye R, Li JY, Lin Y, Gao J, Ren L, Liu B, Jiang L (2018) Touch-actuated microneedle array patch for closed-loop transdermal drug delivery. Drug Deliv 25(1):1728–1739
Joyce JC, Carroll TD, Collins ML, Chen MH, Fritts L, Dutra JC, Rourke TL, Goodson JL, McChesney MB, Prausnitz MR, Rota PA (2018) A microneedle patch for measles and rubella vaccination is immunogenic and protective in infant rhesus macaques. J Infect Dis 218(1):124–132
Maurya A, Nanjappa SH, Honnavar S, Salwa M, Murthy SN (2018) Rapidly dissolving microneedle patches for transdermal iron replenishment therapy. J Pharm Sci US 107(6):1642–1647
Lee JW, Choi SO, Felner EI, Prausnitz MR (2011) Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 7(4):531–539
Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y (2009) Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 27(3):454–459
Zhang Y, Liu Q, Yu J, Yu S, Wang J, Qiang L, Gu Z (2017) Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano 11(9):9223–9230
Kajimura S, Spiegelman BM, Seale P (2015) Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22(4):546–559
Chen X, Kask AS, Crichton ML, McNeilly C, Yukiko S, Dong L, Marshak JO, Jarrahian C, Fernando GJP, Chen D, Koelle DM, Kendall MAF (2010) Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J Control Release 148(3):327–333
Ma Y, Gill HS (2014) Coating solid dispersions on microneedles via a molten dip-coating method: development and in vitro evaluation for transdermal delivery of a water-insoluble drug. J Pharm Sci 103(11):3621–3630
Kumar V, Banga AK (2012) Modulated iontophoretic delivery of small and large molecules through microchannels. Int J Pharm 434(1–2):106–114
Chu LY, Choi S-O, Prausnitz MR (2010) Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci 99(10):4228–4238
Chen M-C, Ling M-H, Lai K-Y, Pramudityo E (2012) Chitosan microneedle patches for sustained transdermal delivery of macromolecules. Biomacromolecules 13(12):4022–4031
Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol 26(11):1261–1268
DeMuth PC, Min Y, Irvine DJ, Hammond PT (2014) Implantable silk composite microneedles for programmable vaccine release kinetics and enhanced immunogenicity in transcutaneous immunization. Adv Healthc Mater 3(1):47–58
Tsioris K, Raja WK, Pritchard EM, Panilaitis B, Kaplan DL, Omenetto FG (2012) Fabrication of silk microneedles for controlled-release drug delivery. Adv Funct Mater 22(2):330–335
Lu Y, Aimetti AA, Langer R, Gu Z (2016) Bioresponsive materials. Nat Rev Mater 2(1):16075
Yu J, Zhang Y, Kahkoska AR, Gu Z (2017) Bioresponsive transcutaneous patches. Curr Opin Biotechnol 48:28–32
Ding X, Stewart MP, Sharei A, Weaver JC, Langer RS, Jensen KF (2017) High-throughput nuclear delivery and rapid expression of DNA via mechanical and electrical cell-membrane disruption. Nat Biomed Eng 1(3):0039
Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J (2016) Nuclear envelope rupture and repair during cancer cell migration. Science 352(6283):353–358
Chiappini C, De Rosa E, Martinez JO, Liu X, Steele J, Stevens MM, Tasciotti E (2015) Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat Mater 14(5):532–539
Xie X, Xu AM, Angle MR, Tayebi N, Verma P, Melosh NA (2013) Mechanical model of vertical nanowire cell penetration. Nano Lett 13(12):6002–6008
Xu AM, Aalipour A, Leal-Ortiz S, Mekhdjian AH, Xie X, Dunn AR, Garner CC, Melosh NA (2014) Quantification of nanowire penetration into living cells. Nat Commun 5(1):3613
Shalek AK, Robinson JT, Karp ES, Lee JS, Ahn D-R, Yoon M-H, Sutton A, Jorgolli M, Gertner RS, Gujral TS, MacBeath G, Yang EG, Park H (2010) Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc Natl Acad Sci USA 107(5):1870–1875
Kim W, Ng JK, Kunitake ME, Conklin BR, Yang P (2007) Interfacing silicon nanowires with mammalian cells. J Am Chem Soc 129(23):7228–7229
Bucaro MA, Vasquez Y, Hatton BD, Aizenberg J (2012) Fine-tuning the degree of stem cell polarization and alignment on ordered arrays of high-aspect-ratio nanopillars. ACS Nano 6(7):6222–6230
Fu J, Wang Y-K, Yang MT, Desai RA, Yu X, Liu Z, Chen CS (2010) Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods 7(9):733–736
Wang Y, Yang Y, Yan L, Kwok SY, Li W, Wang Z, Zhu X, Zhu G, Zhang W, Chen X, Shi P (2014) Poking cells for efficient vector-free intracellular delivery. Nat Commun 5:4466
VanDersarl JJ, Xu AM, Melosh NA (2011) Nanostraws for direct fluidic intracellular access. Nano Lett 12(8):3881–3886
Durney AR, Frenette LC, Hodvedt EC, Krauss TD, Mukaibo H (2016) Fabrication of tapered microtube arrays and their application as a microalgal injection platform. ACS Appl Mater Interfaces 8(50):34198–34208
Fox CB, Cao Y, Nemeth CL, Chirra HD, Chevalier RW, Xu AM, Melosh NA, Desai TA (2016) Fabrication of sealed nanostraw microdevices for oral drug delivery. ACS Nano 10(6):5873–5881
Marshall S, Sahm LJ, Moore AC (2016) The success of microneedle-mediated vaccine delivery into skin. Hum Vaccines Immunother 12(11):2975–2983
Stevenson DJ, Gunn-Moore FJ, Campbell P, Dholakia K (2010) Single cell optical transfection. J R Soc Interface 7(47):863–871
DiTommaso T, Cole JM, Cassereau L, Buggé JA, Hanson JLS, Bridgen DT, Stokes BD, Loughhead SM, Beutel BA, Gilbert JB, Nussbaum K, Sorrentino A, Toggweiler J, Schmidt T, Gyuelveszi G, Bernstein H, Sharei A (2018) Cell engineering with microfluidic squeezing preserves functionality of primary immune cells in vivo. Proc Natl Acad Sci 115(46):E10907–E10914
Kollmannsperger A, Sharei A, Raulf A, Heilemann M, Langer R, Jensen KF, Wieneke R, Tampé R (2016) Live-cell protein labelling with nanometre precision by cell squeezing. Nat Commun 7(1):10372
Acknowledgements
The authors acknowledge the helpful discussions with Dr. Steven J. Jonas and Ms. Isaura M. Frost from University of California Los Angeles.
Funding
X.X. and W.J. acknowledges the support from Tongji University. L.M. acknowledges the support from National Natural Science Foundation of China under Grants 51875518, 81501607 and 51475419, Key Research and Development Projects of Zhejiang Province under Grant 2017C01054.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
WJ, L.M and X.X declare that they have no conflict of interest.
Ethical approval
This review does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jiang, W., Ma, L. & Xu, X. One-dimensional microstructure-assisted intradermal and intracellular delivery. Bio-des. Manuf. 2, 24–30 (2019). https://doi.org/10.1007/s42242-019-00034-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-019-00034-x