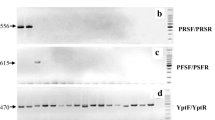
Abstract
Proper diagnosis of the causal agents and their characterization are critical steps in the management of bacterial wilt of tomato and other solanaceous vegetable crops. As the species and phylotypes of Ralstonia are different in different geographical regions, it is important to be able to identify the phylotypes present locally quickly and reliably when identifying sources of resistance to bacterial wilt and when deploying host resistance in different regions. Traditional methods for diagnosis and characterization of the causal agents of bacterial wilt require several steps including pathogen isolation from the infected tissues, culturing in a growth media, genomic DNA extraction from the pathogen culture, and few additional steps for molecular identification. In this study, we used a multiplex PCR method to identify the causal species/phylotypes of the pathogen trapped directly from infected plant tissue on FTA (Flinder Technology Associates) cards. Previously characterized R. pseudosolanacearum strain Pss4 (phylotype I) and R. solanacearum strain Pss1632 (phylotype II) separately were inoculated in tomato and eggplant seedlings. Bacterial ooze either directly squeezed from infected plants or streamed in water or 70% ethanol was directly applied on FTA cards and the samples were amplified using a multiplex polymerase chain reaction (PCR) assay. We evaluated four standard methods for processing bacterial wilt samples on FTA cards, which were rapid, sensitive, and reliable for the identification of the species and phylotypes of R. solanacearum complex. These methods will be useful for the characterization/identification of R. solanacearum complex species and phylotypes from diverse locations worldwide since as yet there is no restriction and no import permit is required for the international movement of bacterial wilt sample DNA immobilized and preserved on FTA cards.





Similar content being viewed by others
References
Cellier G, Prior P (2010) Deciphering phenotypic diversity of Ralstonia solanacearum strains pathogenic to potato. Phytopathology 100:1250–1261
Chandrashekara KN, Prasannakumar MK, Deepa M, Vani A (2012) A rapid, sensitive and reliable method for detecting Ralstonia solanacearum using FTA (Whatman) cards. J Plant Pathol 94:219–221
Fegan M, Prior P (2005) How complex is the Ralstonia solanacearum species complex? In: Allen C, Prior P, Hayward C (eds) Bacterial wilt: the disease and the Ralstonia solanacearum species complex. APS press, St. Paul, pp 449–462
Grund E, Darissa O, Adam G (2010) Application of FTA® cards to sample microbial plant pathogens for PCR and RT-PCR. J Phytopathol 158:750–757
Kubota R, Schell MA, Peckham GD, Rue J, Alvarez AM, Allen C et al (2011) In silico genomic subtraction guides development of highly accurate DNA-based diagnostics for Ralstonia solanacearum race 3 biovar 2 and blood disease bacterium. J Gen Plant Pathol 77:182–193
Lenarčič R, Morisset D, Pirc M, Llop P, Ravnikar M, Dreo T (2014) Loop-mediated isothermal amplification of specific endoglucanase gene sequence for detection of the bacterial wilt pathogen Ralstonia solanacearum. PLoS One 9(4):e96027
Lin C-H, Tsai K-C, Prior P, Wang J-F (2014) Phylogenetic relationships and population structure of Ralstonia solanacearum from diverse origins in Taiwan. Plant Pathol 63:1395–1403
Pastrik KH, Elphinstone JG, Pukall R (2002) Sequence analysis and detection of Ralstonia solanacearum by multiplex PCR amplification of 16S-23S ribosomal intergenic spacer region with internal positive control. Eur J Plant Pathol 108:831–842
Prior P, Fegan M (2005) Recent development in the phylogeny and classification of Ralstonia solanacearum. Acta Hortic 695:127–136
Prior P, Ailloud F, Dalsing BL, Remenant B, Sanchez B, Allen C (2016) Genomic and proteomic evidence supporting the division of the plant pathogen Ralstonia solanacearum into three species. BMC Genomics 17:90
Safni I, Cleenwerck I, De Vos P, Fegan M, Sly L, Kappler U (2014) Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int J Syst Evol Microbiol 64:3087–3103
Scott JW, Wang JF, Hanson P (2005) Breeding tomatoes for resistance to bacterial wilt, a global view. Acta Hortic 695:161–168
Smith LM, Burgoyne LA (2004) Collecting, archiving and processing DNA from wildlife samples using FTA data basing paper. BMC Ecol 4:4
Tran TM, Jacobs JM, Huerta A, Milling A, Weibel J, Allen C (2016) Sensitive, secure detection of race 3 biovar 2 and native U.S. strains of Ralstonia solanacearum. Plant Dis 100:630–639
Weller SA, Elphinstone JG, Smith N, Stead DE, Boonham N (2000) Detection of Ralstonia solanacearum strains using an automated and quantitative flourogenic 50 nuclease TaqMan_ assay. Appl Environ Microbiol 66:2853–2858
Winstead NN, Kelman A (1952) Inoculation techniques for evaluating to Pseudomonas solanacearum. Phytopathology 42:628–634
Wu YF, Lin CH, Wang JF, Cheng AS (2011) Population density of Ralstonia solanacearum potato strain, phylotype II/race 3/biovar 2, and incidence of potato bacterial wilt in fields in Dounan, Yunlin County. Plant Pathol Bullet 20:68–77 [In Chinese]
Acknowledgements
For the research support, we thank core donors to the World Vegetable Center: Republic of China (Taiwan), UK Department for International Development (DFID), United States Agency for International Development (USAID), Australian Centre for International Agricultural Research (ACIAR), Germany, Thailand, Philippines, Korea, and Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Ethical approval
This article does not contain any work conducted on animal or human participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burlakoti, R.R., Hsu, Cf., Chen, Jr. et al. Capture of Ralstonia solanacearum species complex strains directly from plant tissue sampled on FTA cards for molecular characterization. J Plant Pathol 102, 11–17 (2020). https://doi.org/10.1007/s42161-019-00361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-019-00361-z