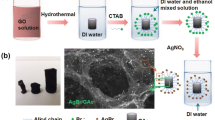
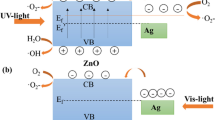
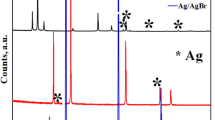
Abstract
The silver halides and their corresponding composites would constitute remarkable photocatalytic systems not only in energy production but also in environmental remediation. The major advantage in these silver halides system is that plasma metal Ag0 species would be spontaneously generated by silver halides under light irradiation, which play dual roles of expanding light absorption range and charge carriers separation. In this tutorial review, the origin and development of silver halides, synthesis methods, construction of composite structures with other materials, photocatalytic applications, and various and controversial mechanisms are all exhaustively described.

The silver halides and their corresponding composites would constitute remarkable photocatalytic systems not only in energy production but also in environmental remediation.













Similar content being viewed by others
References
Wang W-S, Du H, Wang R-X, Wen T, Xu A-W (2013) Heterostructured Ag3PO4/AgBr/Ag plasmonic photocatalyst with enhanced photocatalytic activity and stability under visible light. Nanoscale 5:3315–3321
Martin DJ, Liu G, Moniz SJA, Bi Y, Beale AM, Ye J, Tang J (2015) Efficient visible driven photocatalyst, silver phosphate: performance, understanding and perspective. Chem Soc Rev 44:7808–7828
Cheng Y-J, Yang S-H, Hsu C-S (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109:5868–5923
Chu S, Cui Y, Liu N (2017) The path towards sustainable energy. Nat Mater 16:16–22
Calkins JO, Umasankar Y, O’Neill H, Ramasamy RP (2013) High photo-electrochemical activity of thylakoid-carbon nanotube composites for photosynthetic energy conversion. Energy Environ Sci 6:1891–1900
An C, Peng S, Sun Y (2010) Facile synthesis of sunlight-driven AgCl:Ag plasmonic nanophotocatalyst. Adv Mater 22:2570–2574
Zhao W, Ma W, Chen C, Zhao J, Shuai Z (2004) Efficient degradation of toxic organic pollutants with Ni2O3/TiO2-xBx under visible irradiation. J Am Chem Soc 126:4782–4783
Zberg B, Uggowitzer PJ, Loffler JF (2009) MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat Mater 8:887–891
Wöhrle D, Meissner D (1991) Organic solar cells. Adv Mater 3:129–138
Schultz DM, Yoon TP (2014) Solar synthesis: prospects in visible light photocatalysis. Science 343:980–993
Gratzel M (2001) Photoelectrochemical cells. Nature 414:338–344
Law M, Greene LE, Johnson JC, Saykally R, Yang P (2005) Nanowire dye-sensitized solar cells. Nat Mater 4:455–459
Li G, Shrotriya V, Huang J, Yao Y, Moriarty T, Emery K, Yang Y (2005) High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat Mater 4:864–868
Kim JY, Kim SH, Lee HH, Lee K, Ma W, Gong X, Heeger AJ (2006) New architecture for high-efficiency polymer photovoltaic cells using solution-based titanium oxide as an optical spacer. Adv Mater 18:572–576
Kojima A, Teshima K, Shirai Y, Miyasaka T (2009) Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc 131:6050–6051
Fitzner R, Mena-Osteritz E, Mishra A, Schulz G, Reinold E, Weil M, Korner C, Ziehlke H, Elschner C, Leo K, Riede M, Pfeiffer M, Uhrich C, Bauerle P (2012) Correlation of pi-conjugated oligomer structure with film morphology and organic solar cell performance. J Am Chem Soc 134:11064–11067
Tong L, Iwase A, Nattestad A, Bach U, Weidelener M, Gotz G, Mishra A, Bauerle P, Amal R, Wallace GG, Mozer AJ (2012) Sustained solar hydrogen generation using a dye-sensitised NiO photocathode/BiVO4 tandem photo-electrochemical device. Energy Environ Sci 5:9472–9475
Liu M, Johnston MB, Snaith HJ (2013) Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501:395–398
Jeon NJ, Noh JH, Kim YC, Yang WS, Ryu S, Seok SI (2014) Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat Mater 13:897–903
Mei A, Li X, Liu L, Ku Z, Liu T, Rong Y, Xu M, Hu M, Chen J, Yang Y, Grätzel M, Han H (2014) A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 345:295–298
Wang X, Liow C, Qi D, Zhu B, Leow WR, Wang H, Xue C, Chen X, Li S (2014) Programmable photo-electrochemical hydrogen evolution based on multi-segmented CdS-Au nanorod arrays. Adv Mater 26:3506–3512
Montoya JH, Seitz LC, Chakthranont P, Vojvodic A, Jaramillo TF, Norskov JK (2017) Materials for solar fuels and chemicals. Nat Mater 16:70–81
Wu H, Huang Y, Xu F, Duan Y, Yin Z (2016) Energy harvesters for wearable and stretchable electronics: From flexibility to stretchability. Adv Mater 28:9881–9919
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082
Stamenkovic VR, Strmcnik D, Lopes PP, Markovic NM (2016) Energy and fuels from electrochemical interfaces. Nat Mater 16:57–69
Green MA, Bremner SP (2017) Energy conversion approaches and materials for high-efficiency photovoltaics. Nat Mater 16:23–34
Bredas J-L, Sargent EH, Scholes GD (2017) Photovoltaic concepts inspired by coherence effects in photosynthetic systems. Nat Mater 16:35–44
Zhang G, Choi W (2012) A low-cost sensitizer based on a phenolic resin for charge-transfer type photocatalysts working under visible light. Chem Commun 48:10621–10623
Serpone N, Emeline AV (2012) Semiconductor photocatalysis — past, present, and future outlook. J Phys Chem Lett 3:673–677
Tang J, Liu Y, Li H, Tan Z, Li D (2013) A novel Ag3AsO4 visible-light-responsive photocatalyst: facile synthesis and exceptional photocatalytic performance. Chem Commun 49:5498–5500
Qiu X, Zhao Y, Burda C (2007) Synthesis and characterization of nitrogen-doped group IVB visible-light-photoactive metal oxide nanoparticles. Adv Mater 19:3995–3999
Morales W, Cason M, Aina O, de Tacconi NR, Rajeshwar K (2008) Combustion synthesis and characterization of nanocrystalline WO3. J Am Chem Soc 130:6318–6319
Putri LK, Ong W-J, Chang WS, Chai S-P (2016) Enhancement in the photocatalytic activity of carbon nitride through hybridization with light-sensitive AgCl for carbon dioxide reduction to methane. Catal Sci Technol 6:744–754
Liu J, Zhang G (2014) Recent advances in synthesis and applications of clay-based photocatalysts: a review. Phys Chem Chem Phys 16:8178–8192
Garlisi C, Scandura G, Szlachetko J, Ahmadi S, Sa J, Palmisano G (2016) E-beam evaporated TiO2 and Cu-TiO2 on glass: Performance in the discoloration of methylene blue and 2-propanol oxidation. Appl Catal A Gen 526:191–199
Lou Z, Huang B, Qin X, Zhang X, Wang Z, Zheng Z, Cheng H, Wang P, Dai Y (2011) One-step synthesis of AgBr microcrystals with different morphologies by ILs-assisted hydrothermal method. CrystEngComm 13:1789–1793
Li Y, Zhang H, Guo Z, Han J, Zhao X, Zhao Q, Kim S-J (2008) Highly efficient visible-light-induced photocatalytic activity of nanostructured AgI/TiO2 photocatalyst. Langmuir 24:8351–8357
Khan SUM, Al-Shahry M, Ingler WB (2002) Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297:2243–2245
Liu Z, Hou W, Pavaskar P, Aykol M, Cronin SB (2011) Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett 11:1111–1116
Yi Z, Withers RL, Liu Y (2011) A two-step approach towards solar-driven water splitting. Electrochem Commun 13:28–30
Xie G, Zhang K, Guo B, Liu Q, Fang L, Gong JR (2013) Graphene-based materials for hydrogen generation from light-driven water splitting. Adv Mater 25:3820–3839
Xiang Q, Cheng B, Yu J (2013) Hierarchical porous CdS nanosheet-assembled flowers with enhanced visible-light photocatalytic H2-production performance. Appl Catal B 138–139:299–303
Tang J, Zou Z, Ye J (2004) Efficient photocatalytic decomposition of organic contaminants over CaBi2O4 under visible-light irradiation. Angew Chem Int Ed 43:4463–4466
Li J, Xie Y, Zhong Y, Hu Y (2015) Facile synthesis of Z-scheme Ag2CO3/Ag/AgBr ternary heterostructured nanorods with improved photostability and photoactivity. J Mater Chem A 3:5474–5481
Tong H, Ouyang S, Bi Y, Umezawa N, Oshikiri M, Ye J (2012) Nano-photocatalytic materials: possibilities and challenges. Adv Mater 24:229–251
Wang P, Huang B, Zhang X, Qin X, Jin H, Dai Y, Wang Z, Wei J, Zhan J, Wang S, Wang J, Whangbo M-H (2009) Highly efficient visible-light plasmonic photocatalyst Ag@AgBr. Chem Eur J 15:1821–1824
Sancier KM, Morrison SR (1973) Oxidation of organic molecules by photoproduced holes of ZnO. Surf Sci 36:622–629
Park Y, Lee S-H, Kang SO, Choi W (2010) Organic dye-sensitized TiO2 for the redox conversion of water pollutants under visible light. Chem Commun 46:2477–2479
Kulkarni AA, Bhanage BM (2014) Ag@AgCl nanomaterial synthesis using sugar cane juice and its application in degradation of azo dyes. ACS Sustain Chem Eng 2:1007–1013
Li G, Wong KH, Zhang X, Hu C, Yu JC, Chan RCY, Wong PK (2009) Degradation of Acid Orange 7 using magnetic AgBr under visible light: The roles of oxidizing species. Chemosphere 76:1185–1191
Hu C, Guo J, Qu J, Hu X (2007) Photocatalytic degradation of pathogenic bacteria with AgI/TiO2 under visible light irradiation. Langmuir 23:4982–4987
Akhavan O, Ghaderi E (2009) Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J Phys Chem C 113:20214–20220
Legrini O, Oliveros E, Braun AM (1993) Photochemical processes for water treatment. Chem Rev 93:671–698
Lan Y, Hu C, Hu X, Qu J (2007) Efficient destruction of pathogenic bacteria with AgBr/TiO2 under visible light irradiation. Appl Catal B 73:354–360
Fu Y, Sun D, Chen Y, Huang R, Ding Z, Fu X, Li Z (2012) An amine-functionalized titanium metal–organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew Chem 124:3420–3423
Bozzi A, Yuranova T, Kiwi J (2005) Self-cleaning of wool-polyamide and polyester textiles by TiO2-rutile modification under daylight irradiation at ambient temperature. J Photochem Photobiol A Chem 172:27–34
Kafizas A, Kellici S, Darr JA, Parkin IP (2009) Titanium dioxide and composite metal/metal oxide titania thin films on glass: A comparative study of photocatalytic activity. J Photochem Photobiol A Chem 204:183–190
Protti S, Albini A, Serpone N (2014) Photocatalytic generation of solar fuels from the reduction of H2O and CO2: a look at the patent literature. Phys Chem Chem Phys 16:19790–19827
Tu W, Zhou Y, Zou Z (2014) Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: state-of-the-art accomplishment, challenges, and prospects. Adv Mater 26:4607–4626
Habisreutinger SN, Schmidt-Mende L, Stolarczyk JK (2013) Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew Chem Int Ed 52:7372–7408
An C, Wang J, Jiang W, Zhang M, Ming X, Wang S, Zhang Q (2012) Strongly visible-light responsive plasmonic shaped AgX:Ag (X = Cl, Br) nanoparticles for reduction of CO2 to methanol. Nano 4:5646–5650
Yu H, Shi R, Zhao Y, Waterhouse GIN, Wu L-Z, Tung C-H, Zhang T (2016) Smart utilization of carbon dots in semiconductor photocatalysis. Adv Mater 28:9454–9477
Yu H, Liu L, Wang X, Wang P, Yu J, Wang Y (2012) The dependence of photocatalytic activity and photoinduced self-stability of photosensitive AgI nanoparticles. Dalton Trans 41:10405–10411
Yu C, Li G, Kumar S, Yang K, Jin R (2014) Phase transformation synthesis of novel Ag2O/Ag2CO3 heterostructures with high visible light efficiency in photocatalytic degradation of pollutants. Adv Mater 26:892–898
Yao W, Zhang B, Huang C, Ma C, Song X, Xu Q (2012) Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J Mater Chem 22:4050–4055
Yamashita Y, Gao Y, Yoshida K, Kakuta N, Petrykin V, Kakihana M (2005) Photocatalytic conversion of NO on AgCl/Al2O3 mixed with ZSM-5. J Ceram Soc Jpn 113:509–512
Xu Y, Xu H, Li H, Xia J, Liu C, Liu L (2011) Enhanced photocatalytic activity of new photocatalyst Ag/AgCl/ZnO. J Alloy Compd 509:3286–3292
He K, Zhu K, Chen W (2011) Photocatalytic behavior of PdCl2-modified nanostructured AgI/TiO2 photocatalyst. Rare Metals 30:131–134
Zang Y, Farnood R (2008) Photocatalytic activity of AgBr/TiO2 in water under simulated sunlight irradiation. Appl Catal B 79:334–340
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Bae E, Choi W, Park J, Shin HS, Kim SB, Lee JS (2004) Effects of surface anchoring groups (carboxylate vs phosphonate) in ruthenium-complex-sensitized TiO2 on visible light reactivity in aqueous suspensions. J Phys Chem B 108:14093–14101
Fan Y, Han D, Cai B, Ma W, Javed M, Gan S, Wu T, Siddiq M, Dong X, Niu L (2014) Ce-/S-codoped TiO2/Sulfonated graphene for photocatalytic degradation of organic dyes. J Mater Chem A 2:13565–13570
Tajima T, Sakata W, Wada T, Tsutsui A, Nishimoto S, Miyake M, Takaguchi Y (2011) Photosensitized hydrogen evolution from water using a single-walled carbon nanotube/fullerodendron/SiO2 coaxial nanohybrid. Adv Mater 23:5750–5754
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Klosek S, Raftery D (2001) Visible light driven V-doped TiO2 photocatalyst and its photooxidation of ethanol. J Phys Chem B 105:2815–2819
Zhou X, Liu G, Yu J, Fan W (2012) Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light. J Mater Chem 22:21337–21354
Elahifard MR, Rahimnejad S, Haghighi S, Gholami MR (2007) Apatite-coated Ag/AgBr/TiO2 visible-light photocatalyst for destruction of bacteria. J Am Chem Soc 129:9552–9553
Zhou W, Cao M, Li N, Su S, Zhao X, Wang J, Li X, Hu C (2013) Ag@AgHPW as a plasmonic catalyst for visible-light photocatalytic degradation of environmentally harmful organic pollutants. Mater Res Bull 48:2308–2316
Dombi P, Hörl A, Rácz P, Márton I, Trügler A, Krenn JR, Hohenester U (2013) Ultrafast strong-field photoemission from plasmonic nanoparticles. Nano Lett 13:674–678
Chen H, Shao L, Li Q, Wang J (2013) Gold nanorods and their plasmonic properties. Chem Soc Rev 42:2679–2724
Zhang P, Wang T, Gong J (2015) Understanding of the plasmonic enhancement for solar water splitting. Adv Mater 27:5328–5342
Rycenga M, Cobley CM, Zeng J, Li W, Moran CH, Zhang Q, Qin D, Xia Y (2011) Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem Rev 111:3669–3712
Kuai L, Geng B, Chen X, Zhao Y, Luo Y (2010) Facile subsequently light-induced route to highly efficient and stable sunlight-driven Ag−AgBr plasmonic photocatalyst. Langmuir 26:18723–18727
Chi Y, Zhao L, Lu X, An C, Guo W, Liu Y, Wu C-ML (2015) Effects of subnanometer silver clusters on the AgBr(110) photocatalyst surface: a theoretical investigation. Catal Sci Technol 5:4821–4829
Link S, El-Sayed MA (1999) Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phys Chem B 103:8410–8426
Yang P, Zheng J, Xu Y, Zhang Q and Jiang L (2016) Colloidal synthesis and applications of plasmonic metal nanoparticles. Adv Mater 28:10508–10517
Tsukamoto D, Shiraishi Y, Sugano Y, Ichikawa S, Tanaka S, Hirai T (2012) Gold nanoparticles located at the interface of anatase/rutile TiO2 particles as active plasmonic photocatalysts for aerobic oxidation. J Am Chem Soc 134:6309–6315
Tian Y, Tatsuma T (2005) Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J Am Chem Soc 127:7632–7637
Wang P, Huang B, Dai Y, Whangbo M-H (2012) Plasmonic photocatalysts: harvesting visible light with noble metal nanoparticles. Phys Chem Chem Phys 14:9813–9825
Sun Y (2010) Conversion of Ag nanowires to AgCl nanowires decorated with Au nanoparticles and their photocatalytic activity. J Phys Chem C 114:2127–2133
Xie W, Schlücker S (2015) Hot electron-induced reduction of small molecules on photorecycling metal surfaces. Nat Commun 6:7570
Wang P, Xia Y, Wu P, Wang X, Yu H, Yu J (2014) Cu(II) as a general cocatalyst for improved visible-light photocatalytic performance of photosensitive Ag-based compounds. J Phys Chem C 118:8891–8898
Su R, Tiruvalam R, He Q, Dimitratos N, Kesavan L, Hammond C, Lopez-Sanchez JA, Bechstein R, Kiely CJ, Hutchings GJ, Besenbacher F (2012) Promotion of phenol photodecomposition over TiO2 using Au, Pd, and Au–Pd nanoparticles. ACS Nano 6:6284–6292
Seo JH, Jeon WI, Dembereldorj U, Lee SY, Joo S-W (2011) Cytotoxicity of serum protein-adsorbed visible-light photocatalytic Ag/AgBr/TiO2 nanoparticles. J Hazard Mater 198:347–355
Jin L, Zhu G, Hojamberdiev M, Luo X, Tan C, Peng J, Wei X, Li J, Liu P (2014) A plasmonic Ag–AgBr/Bi2O2CO3 composite photocatalyst with enhanced visible-light photocatalytic activity. Ind Eng Chem Res 53:13718–13727
Jiang R, Li B, Fang C, Wang J (2014) Metal/semiconductor hybrid nanostructures for plasmon-enhanced applications. Adv Mater 26:5274–5309
Colón G, Maicu M, Hidalgo MC, Navío JA (2006) Cu-doped TiO2 systems with improved photocatalytic activity. Appl Catal B 67:41–51
Irie H, Kamiya K, Shibanuma T, Miura S, Tryk DA, Yokoyama T, Hashimoto K (2009) Visible light-sensitive Cu(II)-grafted TiO2 photocatalysts: activities and x-ray absorption fine structure analyses. J Phys Chem C 113:10761–10766
He J, Ichinose I, Kunitake T, Nakao A, Shiraishi Y, Toshima N (2003) Facile fabrication of Ag−Pd bimetallic nanoparticles in ultrathin TiO2-gel films: nanoparticle morphology and catalytic activity. J Am Chem Soc 125:11034–11040
Liu J, Raveendran P, Shervani Z, Ikushima Y, Hakuta Y (2005) Synthesis of Ag and AgI quantum dots in AOT-stabilized water-in-CO2 microemulsions. Chem Eur J 11:1854–1860
Cao J, Xu B, Luo B, Lin H, Chen S (2011) Preparation, characterization and visible-light photocatalytic activity of AgI/AgCl/TiO2. Appl Surf Sci 257:7083–7089
Hirakawa T, Kamat PV (2005) Charge separation and catalytic activity of Ag@TiO2 core−shell composite clusters under UV−irradiation. J Am Chem Soc 127:3928–3934
Montini T, Gombac V, Sordelli L, Delgado JJ, Chen X, Adami G, Fornasiero P (2011) Nanostructured Cu/TiO2 photocatalysts for H2 production from ethanol and glycerol aqueous solutions. ChemCatChem 3:574–577
Li H, Bian Z, Zhu J, Huo Y, Li H, Lu Y (2007) Mesoporous Au/TiO2 nanocomposites with enhanced photocatalytic activity. J Am Chem Soc 129:4538–4539
Xuming Z, Lim CY, Ru-Shi L, Din Ping T (2013) Plasmonic photocatalysis. Rep Prog Phys 76:046401
An C, Wang J, Liu J, Wang S, Zhang Q-H (2014) Plasmonic enhancement of photocatalysis over Ag incorporated AgI hollow nanostructures. RSC Adv 4:2409–2413
An C, Wang S, Sun Y, Zhang Q, Zhang J, Wang C, Fang J (2016) Plasmonic silver incorporated silver halides for efficient photocatalysis. J Mater Chem A 4:4336–4352
Tani T (2007) Review of mechanisms of photographic sensitivity. Imaging Sci J 55:65–79
Smith PV (1976) A tight-binding approach to the electronic structure of the silver halides—II. J Phys Chem Solids 37:589–597
James TH, Kornfeld G (1942) Reduction of silver halides and the mechanism of photographic development. Chem Rev 30:1–32
Emil Baur AR (1921) Über versuche zur photolyse des wassers. Helv Chim Acta 4:256–262
Chandrasekaran K, Thomas JK (1983) The mechanism of the photochemical oxidation of water to oxygen with silver chloride colloids. Chem Phys Lett 97:357–360
Pfanner K, Gfeller N, Calzaferri G (1996) Photochemical oxidation of water with thin AgCl layers. J Photochem Photobiol A Chem 95:175–180
Kakuta N, Goto N, Ohkita H, Mizushima T (1999) Silver bromide as a photocatalyst for hydrogen generation from CH3OH/H2O solution. J Phys Chem B 103:5917–5919
Hu C, Hu X, Wang L, Qu J, Wang A (2006) Visible-light-induced photocatalytic degradation of azodyes in aqueous AgI/TiO2 dispersion. Environ Sci Technol 40:7903–7907
Liang Y, Lin S, Liu L, Hu J, Cui W (2015) Oil-in-water self-assembled Ag@AgCl QDs sensitized Bi2WO6: Enhanced photocatalytic degradation under visible light irradiation. Appl Catal B 164:192–203
Krishnakumar B, Swaminathan M (2012) Photodegradation of acid violet 7 with AgBr–ZnO under highly alkaline conditions. Spectrochim Acta A Mol Biomol Spectrosc 99:160–165
Hu C, Lan Y, Qu J, Hu X, Wang A (2006) Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria. J Phys Chem B 110:4066–4072
Gamage McEvoy J, Cui W, Zhang Z (2014) Synthesis and characterization of Ag/AgCl–activated carbon composites for enhanced visible light photocatalysis. Appl Catal B 144:702–712
Krishnakumar B, Subash B, Swaminathan M (2012) AgBr–ZnO – An efficient nano-photocatalyst for the mineralization of Acid Black 1 with UV light. Sep Purif Technol 85:35–44
Li G, Wang Y, Mao L (2014) Recent progress in highly efficient Ag-based visible-light photocatalysts. RSC Adv 4:53649–53661
Jiang J, Zhang L (2011) Rapid microwave-assisted nonaqueous synthesis and growth mechanism of AgCl/Ag, and its daylight-driven plasmonic photocatalysis. Chem Eur J 17:3710–3717
Li Y, Ding Y (2010) Porous AgCl/Ag nanocomposites with enhanced visible light photocatalytic properties. J Phys Chem C 114:3175–3179
Lin ZY, Xiao J, Yan JH, Liu P, Li LH, Yang GW (2015) Ag/AgCl plasmonic cubes with ultrahigh activity as advanced visible-light photocatalysts for photodegrading dyes. J Mater Chem A 3:7649–7658
Xu Z, Han L, Hu P, Dong S (2014) Facile synthesis of small Ag@AgCl nanoparticles via a vapor diffusion strategy and their highly efficient visible-light-driven photocatalytic performance. Catal Sci Technol 4:3615–3619
Wang D, Duan Y, Luo Q, Li X, Bao L (2011) Visible light photocatalytic activities of plasmonic Ag/AgBr particles synthesized by a double jet method. Desalination 270:174–180
Validžić IL, Janković IA, Mitrić M, Bibić N, Nedeljković JM (2007) Growth and quantum confinement in AgI nanowires. Mater Lett 61:3522–3525
Xu H, Li H, Xia J, Yin S, Luo Z, Liu L, Xu L (2011) One-pot synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl in ionic liquid. ACS Appl Mater Interfaces 3:22–29
Yamada H, Saruwatari I, Kuwata N, Kawamura J (2014) Local structure of thermally stable super ionic conducting AgI confined in mesopores. J Phys Chem C 118:23845–23852
Sanson A, Rocca F, Armellini C, Dalba G, Fornasini P, Grisenti R (2008) Correlation between I-Ag distance and ionic conductivity in AgI fast-ion-conducting glasses. Phys Rev Lett 101:155901
Yamada H, Bhattacharyya AJ, Maier J (2006) Extremely high silver ionic conductivity in composites of silver halide (AgBr, AgI) and mesoporous alumina. Adv Funct Mater 16:525–530
Wu F, Wang W, Xu Z, Li F (2015) Bromide (Br) - based synthesis of Ag nanocubes with high-yield. Sci Rep 5:10772
Li B, Wang H, Zhang B, Hu P, Chen C, Guo L (2013) Facile synthesis of one dimensional AgBr@Ag nanostructures and their visible light photocatalytic properties. ACS Appl Mater Interfaces 5:12283–12287
Ng CHB, Fan WY (2007) Controlled synthesis of β-AgI nanoplatelets from selective nucleation of twinned Ag seeds in a tandem reaction. J Phys Chem C 111:2953–2958
Jiang W, An C, Liu J, Wang S, Zhao L, Guo W, Liu J (2014) Facile aqueous synthesis of [small beta]-AgI nanoplates as efficient visible-light-responsive photocatalyst. Dalton Trans 43:300–305
Bi Y, Ye J (2010) Direct conversion of commercial silver foils into high aspect ratio AgBr nanowires with enhanced photocatalytic properties. Chem Eur J 16:10327–10331
Purbia R, Paria S (2015) Yolk/shell nanoparticles: classifications, synthesis, properties, and applications. Nano 7:19789–19873
Ma X, Dai Y, Lu J, Guo M, Huang B (2012) Tuning of the surface-exposing and photocatalytic activity for AgX (X = Cl and Br): a theoretical study. J Phys Chem C 116:19372–19378
Wang K, Murahari P, Yokoyama K, Lord JS, Pratt FL, He J, Schulz L, Willis M, Anthony JE, Morley NA, Nuccio L, Misquitta A, Dunstan DJ, Shimomura K, Watanabe I, Zhang S, Heathcote P and Drew AJ (2017) Temporal mapping of photochemical reactions and molecular excited states with carbon specificity. Nat Mater 16(4):467–473
Morgan BJ, Madden PA (2011) Effects of lattice polarity on interfacial space charges and defect disorder in ionically conducting AgI heterostructures. Phys Rev Lett 107:206102
Makiura R, Yonemura T, Yamada T, Yamauchi M, Ikeda R, Kitagawa H, Kato K, Takata M (2009) Size-controlled stabilization of the superionic phase to room temperature in polymer-coated AgI nanoparticles. Nat Mater 8:476–480
Wang H, Gao J, Guo T, Wang R, Guo L, Liu Y, Li J (2012) Facile synthesis of AgBr nanoplates with exposed {111} facets and enhanced photocatalytic properties. Chem Commun 48:275–277
Kuang Q, Zheng X, Yang S (2014) AgI microplate monocrystals with polar {0001} facets: spontaneous photocarrier separation and enhanced photocatalytic activity. Chem Eur J 20:2637–2645
Xu Y, Xu H, Li H, Yan J, Xia J, Yin S, Zhang Q (2013) Ionic liquid oxidation synthesis of Ag@AgCl core–shell structure for photocatalytic application under visible-light irradiation. Colloids Surf A Physicochem Eng Asp 416:80–85
Ma B, Guo J, Dai W-L and Fan K (2013) Highly stable and efficient Ag/AgCl core–shell sphere: Controllable synthesis, characterization, and photocatalytic application. Appl Catal B 130–131:257–263
Lou Z, Huang B, Wang P, Wang Z, Qin X, Zhang X, Cheng H, Zheng Z, Dai Y (2011) The synthesis of the near-spherical AgCl crystal for visible light photocatalytic applications. Dalton Trans 40:4104–4110
Zhu M, Chen P, Liu M (2011) Sunlight-driven plasmonic photocatalysts based on Ag/AgCl nanostructures synthesized via an oil-in-water medium: enhanced catalytic performance by morphology selection. J Mater Chem 21:16413–16419
Bashouti MY, Talebi R, Kassar T, Nahal A, Ristein J, Unruh T, Christiansen SH (2016) Systematic surface phase transition of Ag thin films by iodine functionalization at room temperature: evolution of optoelectronic and texture properties. Sci Rep 6:21439
Belloni J, Treguer M, Remita H, De Keyzer R (1999) Enhanced yield of photoinduced electrons in doped silver halide crystals. Nature 402:865–867
Chen D, Yoo SH, Huang Q, Ali G, Cho SO (2012) Sonochemical synthesis of Ag/AgCl nanocubes and their efficient visible-light-driven photocatalytic performance. Chem Eur J 18:5192–5200
Zhang D, Qi L, Ma J, Cheng H (2001) Formation of silver nanowires in aqueous solutions of a double-hydrophilic block copolymer. Chem Mater 13:2753–2755
Zeng J, Zheng Y, Rycenga M, Tao J, Li Z-Y, Zhang Q, Zhu Y, Xia Y (2010) Controlling the shapes of silver nanocrystals with different capping agents. J Am Chem Soc 132:8552–8553
Wiley BJ, Chen Y, McLellan JM, Xiong Y, Li Z-Y, Ginger D, Xia Y (2007) Synthesis and optical properties of silver nanobars and nanorice. Nano Lett 7:1032–1036
An C, Liu J, Wang S, Zhang J, Wang Z, Long R, Sun Y (2014) Concaving AgI sub-microparticles for enhanced photocatalysis. Nano Energy 9:204–211
Chen D, Chen Q, Zhang W, Ge L, Shao G, Fan B, Lu H, Zhang R, Yang D, Shao G (2015) Freeze-dried PVP–Ag+ precursors to novel AgBr/AgCl–Ag hybrid nanocrystals for visible-light-driven photodegradation of organic pollutants. Superlattice Microstruct 80:136–150
Wang H, Qiao X, Chen J, Wang X, Ding S (2005) Mechanisms of PVP in the preparation of silver nanoparticles. Mater Chem Phys 94:449–453
Zhang Z, Zhao B, Hu L (1996) PVP protective mechanism of ultrafine silver powder synthesized by chemical reduction processes. J Solid State Chem 121:105–110
Pastoriza-Santos I, Liz-Marzán LM (2002) Formation of PVP-protected metal nanoparticles in DMF. Langmuir 18:2888–2894
Narayanan R, El-Sayed MA (2004) Changing catalytic activity during colloidal platinum nanocatalysis due to shape changes: electron-transfer reaction. J Am Chem Soc 126:7194–7195
Li X-H, Li J-X, Li G-D, Liu D-P, Chen J-S (2007) Controlled synthesis, growth mechanism, and properties of monodisperse CdS colloidal spheres. Chem Eur J 13:8754–8761
Sun Y, Mayers B, Herricks T, Xia Y (2003) Polyol synthesis of uniform silver nanowires: a plausible growth mechanism and the supporting evidence. Nano Lett 3:955–960
Chen D, Liu M, Chen Q, Ge L, Fan B, Wang H, Lu H, Yang D, Zhang R, Yan Q, Shao G, Sun J, Gao L (2014) Large-scale synthesis and enhanced visible-light-driven photocatalytic performance of hierarchical Ag/AgCl nanocrystals derived from freeze-dried PVP–Ag+ hybrid precursors with porosity. Appl Catal B 144:394–407
Cai B, Wang J, Gan S, Han D, Wu Z, Niu L (2014) A distinctive red Ag/AgCl photocatalyst with efficient photocatalytic oxidative and reductive activities. J Mater Chem A 2:5280–5286
Han L, Wang P, Zhu C, Zhai Y, Dong S (2011) Facile solvothermal synthesis of cube-like Ag@AgCl: a highly efficient visible light photocatalyst. Nano 3:2931–2935
Han J, Liu Y, Guo R (2009) Reactive template method to synthesize gold nanoparticles with controllable size and morphology supported on shells of polymer hollow microspheres and their application for aerobic alcohol oxidation in water. Adv Funct Mater 19:1112–1117
Aldaye FA, Sleiman HF (2006) Sequential self-assembly of a DNA hexagon as a template for the organization of gold nanoparticles. Angew Chem Int Ed 45:2204–2209
Wang G, Mitomo H, Matsuo Y, Shimamoto N, Niikura K, Ijiro K (2013) DNA-templated plasmonic Ag/AgCl nanostructures for molecular selective photocatalysis and photocatalytic inactivation of cancer cells. J Mater Chem B 1:5899–5907
Yan Z, Compagnini G, Chrisey DB (2011) Generation of AgCl cubes by excimer laser ablation of bulk Ag in aqueous NaCl solutions. J Phys Chem C 115:5058–5062
Xiong W, Zhao Q, Li X, Zhang D (2011) One-step synthesis of flower-like Ag/AgCl/BiOCl composite with enhanced visible-light photocatalytic activity. Catal Commun 16:229–233
Sun C, Chen P, Zhou S (2007) AgCl nanoparticle nanowires fabricated by template method. Mater Lett 61:1645–1648
Lee W, Yoo H.-I and Lee J.-K (2001) Template route toward a novel nanostructured superionic conductor film; AgI nanorod/γ-Al2O3. Chem Commun 0(24):2530–2531
Piao Y and Kim H (2003) Paired cell for the preparation of AgI nanowires using nanoporous alumina membrane templates. Chem Commun 9:2898–2899
Zhang H, Tsuchiya T, Liang C, Terabe K (2015) Size-controlled AgI/Ag heteronanowires in highly ordered alumina membranes: superionic phase stabilization and conductivity. Nano Lett 15:5161–5167
El-Kouedi M, Foss CA, Bodolosky-Bettis SA, Bachman RE (2002) Structural analysis of AgI and Au/AgI nanocomposite films by powder x-ray diffraction: evidence for preferential orientation. J Phys Chem B 106:7205–7209
Li H, Wu T, Cai B, Ma W, Sun Y, Gan S, Han D and Niu L (2015) Efficiently photocatalytic reduction of carcinogenic contaminant Cr (VI) upon robust AgCl:Ag hollow nanocrystals. Appl Catal B 164:344–351
Han C, Ge L, Chen C, Li Y, Zhao Z, Xiao X, Li Z, Zhang J (2014) Site-selected synthesis of novel Ag@AgCl nanoframes with efficient visible light induced photocatalytic activity. J Mater Chem A 2:12594–12600
Xiao X, Ge L, Han C, Li Y, Zhao Z, Xin Y, Fang S, Wu L, Qiu P (2015) A facile way to synthesize Ag@AgBr cubic cages with efficient visible-light-induced photocatalytic activity. Appl Catal B 163:564–572
Kurihara K, Kizling J, Stenius P, Fendler JH (1983) Laser and pulse radiolytically induced colloidal gold formation in water and in water-in-oil microemulsions. J Am Chem Soc 105:2574–2579
Zarur AJ, Ying JY (2000) Reverse microemulsion synthesis of nanostructured complex oxides for catalytic combustion. Nature 403:65–67
Yoon B, Wai CM (2005) Microemulsion-templated synthesis of carbon nanotube-supported Pd and Rh nanoparticles for catalytic applications. J Am Chem Soc 127:17174–17175
Vaucher S, Fielden J, Li M, Dujardin E, Mann S (2002) Molecule-based magnetic nanoparticles: synthesis of cobalt hexacyanoferrate, cobalt pentacyanonitrosylferrate, and chromium hexacyanochromate coordination polymers in water-in-oil microemulsions. Nano Lett 2:225–229
Mo Z, Zuo D, Chen H, Sun Y, Zhang P (2007) Synthesis of graphite nanosheets/AgCl/polypyrrole composites via two-step inverse microemulsion method. Eur Polym J 43:300–306
Husein MM, Rodil E, Vera JH (2006) A novel approach for the preparation of AgBr nanoparticles from their bulk solid precursor using CTAB microemulsions. Langmuir 22:2264–2272
Husein MM, Rodil E, Vera JH (2005) A novel method for the preparation of silver chloride nanoparticles starting from their solid powder using microemulsions. J Colloid Interface Sci 288:457–467
Jones MR, Osberg KD, Macfarlane RJ, Langille MR, Mirkin CA (2011) Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem Rev 111:3736–3827
Hoar TP and Schulman JH (1943) Transparent water-in-oil disperdions: the oleopathic hydro-micelle. Nature 152:102
Schulman JH, Stoeckenius W, Prince LM (1959) Mechanism of formation and structure of Micro emulsions by electron microscopy. J Phys Chem 63:1677–1680
Xu S, Li Y (2003) Different morphology at different reactant molar ratios: synthesis of silver halide low-dimensional nanomaterials in microemulsions. J Mater Chem 13:163–165
Jana NR, Gearheart L, Murphy CJ (2001) Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater 13:1389–1393
Liu Y, Chu Y, Yang L, Han D, Lü Z (2005) A novel solution-phase route for the synthesis of crystalline silver nanowires. Mater Res Bull 40:1796–1801
Ni C, Hassan PA, Kaler EW (2005) Structural characteristics and growth of pentagonal silver nanorods prepared by a surfactant method. Langmuir 21:3334–3337
Vaidya S, Rastogi P, Agarwal S, Gupta SK, Ahmad T, Antonelli AM, Ramanujachary KV, Lofland SE, Ganguli AK (2008) Nanospheres, nanocubes, and nanorods of nickel oxalate: control of shape and size by surfactant and solvent. J Phys Chem C 112:12610–12615
Zheng X, Zhu L, Yan A, Wang X, Xie Y (2003) Controlling synthesis of silver nanowires and dendrites in mixed surfactant solutions. J Colloid Interface Sci 268:357–361
Zhang J, Han B, Liu M, Liu D, Dong Z, Liu J, Li D, Wang J, Dong B, Zhao H, Rong L (2003) Ultrasonication-induced formation of silver nanofibers in reverse micelles and small-angle x-ray scattering studies. J Phys Chem B 107:3679–3683
Wang D, Song C, Hu Z, Zhou X (2005) Synthesis of silver nanoparticles with flake-like shapes. Mater Lett 59:1760–1763
Maillard M, Giorgio S, Pileni M-P (2003) Tuning the size of silver nanodisks with similar aspect ratios: synthesis and optical properties. J Phys Chem B 107:2466–2470
Doruk JS, Yener O, Randall CA, Adair JH (2002) Synthesis of nanosized silver platelets in octylamine-water bilayer systems. Langmuir 18:8692–8699
Zheng X, Zhu L, Wang X, Yan A, Xie Y (2004) A simple mixed surfactant route for the preparation of noble metal dendrites. J Cryst Growth 260:255–262
Schwuger M-J, Stickdorn K, Schomaecker R (1995) Microemulsions in technical processes. Chem Rev 95:849–864
Debuigne F, Jeunieau L, Wiame M, Nagy JB (2000) Synthesis of organic nanoparticles in different W/O microemulsions. Langmuir 16:7605–7611
Ohde H, Rodriguez J M, Ye X-R and Wai C M (2000) Synthesizing silver halide nanoparticles in supercritical carbon dioxide utilizing a water-in-CO2 microemulsion. Chem Commun 23:2353–2354
Husein M, Rodil E, Vera J (2003) Formation of silver chloride nanoparticles in microemulsions by direct precipitation with the surfactant counterion. Langmuir 19:8467–8474
Sugimoto T, Kimijima KI (2003) New approach to the formation mechanism of AgCl nanoparticles in a reverse micelle system. J Phys Chem B 107:10753–10759
Kimijima KI, Sugimoto T (2004) Growth mechanism of AgCl nanoparticles in a reverse micelle system. J Phys Chem B 108:3735–3738
Zhu M, Chen P, Liu M (2011) Graphene oxide enwrapped Ag/AgX (X = Br, Cl) nanocomposite as a highly efficient visible-light plasmonic photocatalyst. ACS Nano 5:4529–4536
Zhu M, Chen P, Liu M (2012) Ag/AgBr/graphene oxide nanocomposite synthesized via oil/water and water/iil microemulsions: a comparison of sunlight energized plasmonic photocatalytic activity. Langmuir 28:3385–3390
Fan Y, Ma W, Han D, Gan S, Dong X, Niu L (2015) Convenient recycling of 3D AgX/graphene aerogels (X = Br, Cl) for efficient photocatalytic degradation of water pollutants. Adv Mater 27:3767–3773
Cai B, Wang J, Han D, Gan S, Zhang Q, Wu Z, Niu L (2013) Ternary alloyed AgCl(x)Br(1-x) nanocrystals: facile modulation of electronic structures toward advancedphotocatalytic performance. Nano 5:10989–10995
Wang X, Yang J, Ma S, Zhao D, Dai J, Zhang D (2016) In situ fabrication of AgI/AgVO3 nanoribbon composites with enhanced visible photocatalytic activity for redox reactions. Catal Sci Technol 6:243–253
Shen C-C, Zhu Q, Zhao Z-W, Wen T, Wang X, Xu A-W (2015) Plasmon enhanced visible light photocatalytic activity of ternary Ag2Mo2O7@AgBr-Ag rod-like heterostructures. J Mater Chem A 3:14661–14668
Yang M, Zhou K (2011) Synthesis and characterizations of spherical hollow composed of AgI nanoparticle using AgBr as the precursor. Appl Surf Sci 257:2503–2507
Abou Asi M, He C, Su M, Xia D, Lin L, Deng H, Xiong Y, Qiu R, Li X-z (2011) Photocatalytic reduction of CO2 to hydrocarbons using AgBr/TiO2 nanocomposites under visible light. Catal Today 175:256–263
Wang P, Huang B, Zhang Q, Zhang X, Qin X, Dai Y, Zhan J, Yu J, Liu H, Lou Z (2010) Highly efficient visible light plasmonic photocatalyst Ag@Ag(Br,I). Chem Eur J 16:10042–10047
Li J, Yang W, Ning J, Zhong Y, Hu Y (2014) Rapid formation of AgnX(X = S, Cl, PO4, C2O4) nanotubes via an acid-etching anion exchange reaction. Nano 6:5612–5615
An C, Wang J, Qin C, Jiang W, Wang S, Li Y, Zhang Q (2012) Synthesis of Ag@AgBr/AgCl heterostructured nanocashews with enhanced photocatalytic performance via anion exchange. J Mater Chem 22:13153–13158
Choi WS, Byun GY, Bae TS, Lee H-J (2013) Evolution of AgX nanowires into Ag derivative nano/microtubes for highly efficient visible-light photocatalysts. ACS Appl Mater Interfaces 5:11225–11233
Lou S, Jia X, Wang Y, Zhou S (2015) Template-assisted in-situ synthesis of porous AgBr/Ag composite microspheres as highly efficient visible-light photocatalyst. Appl Catal B 176–177:586–593
Cheng H, Huang B, Wang P, Wang Z, Lou Z, Wang J, Qin X, Zhang X, Dai Y (2011) In situ ion exchange synthesis of the novel Ag/AgBr/BiOBr hybrid with highly efficient decontamination of pollutants. Chem Commun 47:7054–7056
Bai S, Li X, Kong Q, Long R, Wang C, Jiang J, Xiong Y (2015) Toward enhanced photocatalytic oxygen evolution: synergetic utilization of plasmonic effect and schottky junction via interfacing facet selection. Adv Mater 27:3444–3452
Berry CR (1967) Effects of crystal surface on the optical absorption edge of AgBr. Phys Rev 153:989–992
Wang P, Huang B, Lou Z, Zhang X, Qin X, Dai Y, Zheng Z, Wang X (2010) Synthesis of highly efficient Ag@AgCl plasmonic photocatalysts with various structures. Chem Eur J 16:538–544
Tachikawa T, Yamashita S, Majima T (2011) Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J Am Chem Soc 133:7197–7204
Yin Y, Alivisatos AP (2005) Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature 437:664–670
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102
Ardo S, Meyer GJ (2009) Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem Soc Rev 38:115–164
Ma X, Dai Y, Guo M, Zhu Y, Huang B (2013) Insights into the adsorption and energy transfer of Ag clusters on the AgCl(100) surface. Phys Chem Chem Phys 15:8722–8731
Kisch H (2013) Semiconductor photocatalysis—mechanistic and synthetic aspects. Angew Chem Int Ed 52:812–847
Bi Y, Ouyang S, Cao J, Ye J (2011) Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys Chem Chem Phys 13:10071–10075
Huo P, Yan Y, Li S, Li H, Huang W (2010) Floating photocatalysts of fly-ash cenospheres supported AgCl/TiO2 films with enhanced Rhodamine B photodecomposition activity. Desalination 256:196–200
Yang HG, Sun CH, Qiao SZ, Zou J, Liu G, Smith SC, Cheng HM, Lu GQ (2008) Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453:638–641
Jang ES, Won JH, Hwang SJ, Choy JH (2006) Fine tuning of the face orientation of ZnO crystals to optimize their photocatalytic activity. Adv Mater 18:3309–3312
Liu G, Yu JC, Lu GQ, Cheng H-M (2011) Crystal facet engineering of semiconductor photocatalysts: motivations, advances and unique properties. Chem Commun 47:6763–6783
Zhang H, Lu Y, Liu H, Fang J (2015) One-pot synthesis of high-index faceted AgCl nanocrystals with trapezohedral, concave hexoctahedral structures and their photocatalytic activity. Nano 7:11591–11601
Chen J, Lim B, Lee EP, Xia Y (2009) Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 4:81–95
Xia Y, Xiong Y, Lim B, Skrabalak SE (2009) Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew Chem Int Ed 48:60–103
Wang H, Yang J, Li X, Zhang H, Li J, Guo L (2012) Facet-dependent photocatalytic properties of AgBr nanocrystals. Small 8:2802–2806
Wang H, Lang X, Gao J, Liu W, Wu D, Wu Y, Guo L, Li J (2012) Polyhedral AgBr microcrystals with an increased percentage of exposed {111} facets as a highly efficient visible-light photocatalyst. Chem Eur J 18:4620–4626
Pica M, Nocchetti M, Ridolfi B, Donnadio A, Costantino F, Gentili PL, Casciola M (2015) Nanosized zirconium phosphate/AgCl composite materials: a new synergy for efficient photocatalytic degradation of organic dye pollutants. J Mater Chem A 3:5525–5534
Zhao Y, Kuai L, Geng B (2012) Low-cost and highly efficient composite visible light-driven Ag-AgBr/[gamma]-Al2O3 plasmonic photocatalyst for degrading organic pollutants. Catal. Sci. Technol. 2:1269–1274
Guo J-F, Ma B, Yin A, Fan K, Dai W-L (2012) Highly stable and efficient Ag/AgCl@TiO2 photocatalyst: preparation, characterization, and application in the treatment of aqueous hazardous pollutants. J Hazard Mater 211–212:77–82
Cheng H, Wang W, Huang B, Wang Z, Zhan J, Qin X, Zhang X, Dai Y (2013) Tailoring AgI nanoparticles for the assembly of AgI/BiOI hierarchical hybrids with size-dependent photocatalytic activities. J Mater Chem A 1:7131–7136
Lou Z, Huang B, Qin X, Zhang X, Cheng H, Liu Y, Wang S, Wang J, Dai Y (2012) One-step synthesis of AgCl concave cubes by preferential overgrowth along <111> and <110> directions. Chem Commun 48:3488–3490
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959
Yi Z, Ye J, Kikugawa N, Kako T, Ouyang S, Stuart-Williams H, Yang H, Cao J, Luo W, Li Z, Liu Y, Withers RL (2010) An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat Mater 9:559–564
Ouyang S, Kikugawa N, Chen D, Zou Z, Ye J (2009) A systematical study on photocatalytic properties of AgMO2 (M = Al, Ga, In): effects of chemical compositions, crystal structures, and electronic structures. J Phys Chem C 113:1560–1566
Chen X, Burda C (2008) The electronic origin of the visible-light absorption properties of C-, N- and S-Doped TiO2 nanomaterials. J Am Chem Soc 130:5018–5019
Hosogi Y, Kato H, Kudo A (2008) Photocatalytic activities of layered titanates and niobates Ion-exchanged with Sn2+ under visible light irradiation. J Phys Chem C 112:17678–17682
Nasir M, Xi Z, Xing M, Zhang J, Chen F, Tian B, Bagwasi S (2013) Study of synergistic effect of Ce- and S-codoping on the enhancement of visible-light photocatalytic activity of TiO2. J Phys Chem C 117:9520–9528
Ouyang S, Ye J (2011) β-AgAl1-xGaxO2 solid-solution photocatalysts: continuous modulation of electronic structure toward high-performance visible-light photoactivity. J Am Chem Soc 133:7757–7763
Maeda K, Teramura K, Lu D, Takata T, Saito N, Inoue Y, Domen K (2006) Photocatalyst releasing hydrogen from water. Nature 440:295–295
Gotlib IY, Ivanov-Schitz AK, Murin IV, Petrov AV, Zakalyukin RM (2012) Structure and ionic transport properties of AgI1–xBrx within single-wall carbon nanotubes from molecular dynamics simulation. J Phys Chem C 116:19554–19570
Tang Y, Jiang Z, Xing G, Li A, Kanhere PD, Zhang Y, Sum TC, Li S, Chen X, Dong Z, Chen Z (2013) Efficient Ag@AgCl cubic cage photocatalysts profit from ultrafast plasmon-induced electron transfer processes. Adv Funct Mater 23:2932–2940
Wang P, Huang B, Zhang X, Qin X, Dai Y, Wang Z, Lou Z (2011) Highly efficient visible light plasmonic photocatalysts Ag@Ag(Cl,Br) and Ag@AgCl-AgI. ChemCatChem 3:360–364
Zhang Z, Yates JT (2012) Band bending in semiconductors: chemical and physical consequences at surfaces and interfaces. Chem Rev 112:5520–5551
Chen X, Shen S, Guo L, Mao SS (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev 110:6503–6570
Yang Y, Zhang G, Xu W (2012) Facile synthesis and photocatalytic properties of AgAgClTiO2/rectorite composite. J Colloid Interface Sci 376:217–223
Wang P, Huang B, Qin X, Zhang X, Dai Y, Whangbo M-H (2009) Ag/AgBr/WO3·H2O: visible-light photocatalyst for bacteria destruction. Inorg Chem 48:10697–10702
Wang X, Li S, Ma Y, Yu H, Yu J (2011) H2WO4·H2O/Ag/AgCl composite nanoplates: a plasmonic Z-scheme visible-light photocatalyst. J Phys Chem C 115:14648–14655
Sun W, Li Y, Shi W, Zhao X, Fang P (2011) Formation of AgI/TiO2 nanocomposite leads to excellent thermochromic reversibility and photostability. J Mater Chem 21:9263–9270
Wang P, Huang B, Zhang X, Qin X, Dai Y, Jin H, Wei J, Whangbo M-H (2008) Composite semiconductor H2WO4⋅H2O/AgCl as an efficient and stable photocatalyst under visible light. Chem Eur J 14:10543–10546
Vignesh K, Suganthi A, Rajarajan M, Sara SA (2012) Photocatalytic activity of AgI sensitized ZnO nanoparticles under visible light irradiation. Powder Technol 224:331–337
Tada H, Mitsui T, Kiyonaga T, Akita T, Tanaka K (2006) All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system. Nat Mater 5:782–786
Tian B, Dong R, Zhang J, Bao S, Yang F, Zhang (2014) Sandwich-structured AgCl@Ag@TiO2 with excellent visible-light photocatalytic activity for organic pollutant degradation and E. coli K12 inactivation. J Appl Catal B 158–159:76–84
Hou J, Wang Z, Yang C, Zhou W, Jiao S, Zhu H (2013) Hierarchically plasmonic Z-scheme photocatalyst of Ag/AgCl nanocrystals decorated mesoporous single-crystalline metastable Bi20TiO32 nanosheets. J Phys Chem C 117:5132–5141
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93:341–357
Yu J, Dai G, Huang B (2009) Fabrication and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanotube arrays. J Phys Chem C 113:16394–16401
Jahurul Islam M, Amaranatha Reddy D, Han NS, Choi J, Song JK, Kim TK (2016) An oxygen-vacancy rich 3D novel hierarchical MoS2/BiOI/AgI ternary nanocomposite: enhanced photocatalytic activity through photogenerated electron shuttling in a Z-scheme manner. Phys Chem Chem Phys 18:24984–24993
Wan Z, Zhang G (2015) Synthesis and facet-dependent enhanced photocatalytic activity of Bi2SiO5/AgI nanoplate photocatalysts. J Mater Chem A 3:16737–16745
Chen D, Li T, Chen Q, Gao J, Fan B, Li J, Li X, Zhang R, Sun J, Gao L (2012) Hierarchically plasmonic photocatalysts of Ag/AgCl nanocrystals coupled with single-crystalline WO3 nanoplates. Nano 4:5431–5439
Zhou Z, Long M, Cai W, Cai J (2012) Synthesis and photocatalytic performance of the efficient visible light photocatalyst Ag–AgCl/BiVO4. J Mol Catal A Chem 353–354:22–28
Feng N, Wang Q, Zheng A, Zhang Z, Fan J, Liu S-B, Amoureux J-P, Deng F (2013) Understanding the high photocatalytic activity of (B, Ag)-codoped TiO2 under solar-light irradiation with XPS, solid-state NMR, and DFT calculations. J Am Chem Soc 135:1607–1616
Zhou J, Cheng Y, Yu J (2011) Preparation and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanocomposite thin films. J Photochem Photobiol A Chem 223:82–87
Zhang Y, Tang Z-R, Fu X, Xu Y-J (2011) Nanocomposite of Ag–AgBr–TiO2 as a photoactive and durable catalyst for degradation of volatile organic compounds in the gas phase. Appl Catal B 106:445–452
Cheng H, Huang B, Dai Y, Qin X, Zhang X (2010) One-step synthesis of the nanostructured AgI/BiOI composites with highly enhanced visible-light photocatalytic performances. Langmuir 26:6618–6624
Wu D, Long M (2011) Enhancing visible-light activity of the self-cleaning TiO2-coated cotton fabrics by loading AgI particles. Surf Coat Technol 206:1175–1179
Begum G, Manna J, Rana RK (2012) Controlled orientation in a bio-inspired assembly of Ag/AgCl/ZnO nanostructures enables enhancement in visible-light-induced photocatalytic performance. Chem Eur J 18:6847–6853
Cao J, Luo B, Lin H, Xu B, Chen S (2012) Visible light photocatalytic activity enhancement and mechanism of AgBr/Ag3PO4 hybrids for degradation of methyl orange. J Hazard Mater 217–218:107–115
Zhang L-S, Wong K-H, Yip H-Y, Hu C, Yu JC, Chan C-Y, Wong P-K (2010) Effective photocatalytic disinfection of E. coli K-12 using AgBr−Ag−Bi2WO6 nanojunction system irradiated by visible light: the role of diffusing hydroxyl Radicals. Environ Sci Technol 44:1392–1398
Cao J, Luo B, Lin H, Chen S (2011) Synthesis, characterization and photocatalytic activity of AgBr/H2WO4 composite photocatalyst. J Mol Catal A Chem 344:138–144
Zhang L, Wong K-H, Chen Z, Yu JC, Zhao J, Hu C, Chan C-Y, Wong P-K (2009) AgBr-Ag-Bi2WO6 nanojunction system: a novel and efficient photocatalyst with double visible-light active components. Appl Catal A Gen 363:221–229
Cao J, Luo B, Lin H, Chen S (2011) Photocatalytic activity of novel AgBr/WO3 composite photocatalyst under visible light irradiation for methyl orange degradation. J Hazard Mater 190:700–706
Marchetti AP, Muenter AA, Baetzold RC, McCleary RT (1998) Formation and spectroscopic manifestation of silver clusters on silver bromide surfaces. J Phys Chem B 102:5287–5297
Ma X, Dai Y, Guo M, Huang B (2012) The role of effective mass of carrier in the photocatalytic behavior of silver halide-based Ag@AgX (X=Cl, Br, I): a theoretical study. ChemPhysChem 13:2304–2309
Linic S, Christopher P, Ingram DB (2011) Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat Mater 10:911–921
Lan J, Zhou X, Liu G, Yu J, Zhang J, Zhi L, Nie G (2011) Enhancing photocatalytic activity of one-dimensional KNbO3 nanowires by Au nanoparticles under ultraviolet and visible-light. Nano 3:5161–5167
Hailstone RK (1995) Computer simulation studies of Silver cluster formation on AgBr microcrystals. J Phys Chem 99:4414–4428
Andersson M, Birkedal H, Franklin NR, Ostomel T, Boettcher S, Palmqvist AEC, Stucky GD (2005) Ag/AgCl-loaded ordered mesoporous anatase for photocatalysis. Chem Mater 17:1409–1415
Shah ZH, Wang J, Ge Y, Wang C, Mao W, Zhang S, Lu R (2015) Highly enhanced plasmonic photocatalytic activity of Ag/AgCl/TiO2 by CuO co-catalyst. J Mater Chem A 3:3568–3575
Yang L, Wang F, Shu C, Liu P, Zhang W, Hu S (2016) An in-situ synthesis of Ag/AgCl/TiO2/hierarchical porous magnesian material and its photocatalytic performance. Sci Rep 6:21617
Wang X, Tang Y, Chen Z, Lim T-T (2012) Highly stable heterostructured Ag-AgBr/TiO2 composite: a bifunctional visible-light active photocatalyst for destruction of ibuprofen and bacteria. J Mater Chem 22:23149–23158
Tian G, Chen Y, Bao H-L, Meng X, Pan K, Zhou W, Tian C, Wang J-Q, Fu H (2012) Controlled synthesis of thorny anatase TiO2 tubes for construction of Ag-AgBr/TiO2 composites as highly efficient simulated solar-light photocatalyst. J Mater Chem 22:2081–2088
An C, Jiang W, Wang J, Wang S, Ma Z, Li Y (2013) Synthesis of three-dimensional AgI@TiO2 nanoparticles with improved photocatalytic performance. Dalton Trans 42:8796–8801
Hou Y, Li X, Zhao Q, Quan X, Chen G (2011) TiO2 nanotube/Ag-AgBr three-component nanojunction for efficient photoconversion. J Mater Chem 21:18067–18076
Ma B, Guo J, Dai W-L, Fan K (2012) Ag-AgCl/WO3 hollow sphere with flower-like structure and superior visible photocatalytic activity. Appl Catal B 123–124:193–199
Li H, Sun Y, Cai B, Gan S, Han D, Niu L, Wu T (2015) Hierarchically Z-scheme photocatalyst of Ag@AgCl decorated on BiVO4 (0 4 0) with enhancing photoelectrochemical and photocatalytic performance. Appl Catal B 170–171:206–214
Wu C, Shen L, Zhang YC, Huang Q (2012) Synthesis of AgBr/ZnO nanocomposite with visible light-driven photocatalytic activity. Mater Lett 66:83–85
Yu D, Bai J, Liang H, Wang J, Li C (2015) Fabrication of a novel visible-light-driven photocatalyst Ag-AgI-TiO2 nanoparticles supported on carbon nanofibers. Appl Surf Sci 349:241–250
Wang D, Duan Y, Luo Q, Li X, An J, Bao L, Shi L (2012) Novel preparation method for a new visible light photocatalyst: mesoporous TiO2 supported Ag/AgBr. J Mater Chem 22:4847–4854
Li H, Gan S, Wang H, Han D, Niu L (2015) Intercorrelated superhybrid of AgBr supported on graphitic-C3N4-decorated nitrogen-doped graphene: high engineering photocatalytic activities for water purification and CO2 reduction. Adv Mater 27:6906–6913
Zhou P, Yu J, Jaroniec M (2014) All-solid-state Z-scheme photocatalytic systems. Adv Mater 26:4920–4935
Zhang S, Li J, Wang X, Huang Y, Zeng M, Xu J (2014) In situ ion exchange synthesis of strongly coupled Ag@AgCl/g-C3N4 porous nanosheets as plasmonic photocatalyst for highly efficient visible-light photocatalysis. ACS Appl Mater Interfaces 6:22116–22125
Akhundi A, Habibi-Yangjeh A (2015) Ternary g-C3N4/ZnO/AgCl nanocomposites: Synergistic collaboration on visible-light-driven activity in photodegradation of an organic pollutant. Appl Surf Sci 358(Part A):261–269
Xu H, Yan J, Xu Y, Song Y, Li H, Xia J, Huang C, Wan H (2013) Novel visible-light-driven AgX/graphite-like C3N4 (X = Br, I) hybrid materials with synergistic photocatalytic activity. Appl Catal B 129:182–193
Yang Y, Guo W, Guo Y, Zhao Y, Yuan X, Guo Y (2014) Fabrication of Z-scheme plasmonic photocatalyst Ag@AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. J Hazard Mater 271:150–159
Dong C, Wu K-L, Wei X-W, Wang J, Liu L, Jiang B-B (2014) Nitrogen-doped graphene modified AgX@Ag (X = Br, Cl) composites with improved visible light photocatalytic activity and stability. Appl Catal A Gen 488:11–18
Zhang N, Zhang Y, Xu Y-J (2012) Recent progress on graphene-based photocatalysts: current status and future perspectives. Nano 4:5792–5813
Zhang H, Lv X, Li Y, Wang Y, Li J (2010) P25-graphene composite as a high performance photocatalyst. ACS Nano 4:380–386
Wang Y, Sun L, Fugetsu B (2013) Morphology-controlled synthesis of sunlight-driven plasmonic photocatalysts Ag@AgX (X = Cl, Br) with graphene oxide template. J Mater ChemA 1:12536–12544
Sohrabnezhad S, Pourahmad A (2012) AgBr/Al-MCM-41 visible-light photocatalyst for gas-phase decomposition of CH3CHO. Spectrochim Acta A Mol Biomol Spectrosc 86:271–275
Rodrigues S, Uma S, Martyanov IN, Klabunde KJ (2005) AgBr/Al-MCM-41 visible-light photocatalyst for gas-phase decomposition of CH3CHO. J Catal 233:405–410
Reddy VR, Currao A, Calzaferri G (2007) Zeolite A and zeolite L monolayers modified with AgCl as photocatalyst for water oxidation to O2. J Mater Chem 17:3603–3609
Qu Y, Duan X (2013) Progress, challenge and perspective of heterogeneous photocatalysts. Chem Soc Rev 42:2568–2580
Tang Y, Jiang Z, Deng J, Gong D, Lai Y, Tay HT, Joo IT, Lau TH, Dong Z, Chen Z (2012) Synthesis of nanostructured silver/silver halides on titanate surfaces and their visible-light photocatalytic performance. ACS Appl Mater Interfaces 4:438–446
Tang Y, Subramaniam VP, Lau TH, Lai Y, Gong D, Kanhere PD, Cheng YH, Chen Z, Dong Z (2011) In situ formation of large-scale Ag/AgCl nanoparticles on layered titanate honeycomb by gas phase reaction for visible light degradation of phenol solution. Appl Catal B 106:577–585
Pourahmad A, Sohrabnezhad S, Kashefian E (2010) AgBr/nanoAlMCM-41 visible light photocatalyst for degradation of methylene blue dye. Spectrochim Acta A Mol Biomol Spectrosc 77:1108–1114
Song S, Hong F, He Z, Cai Q, Chen J (2012) AgIO3-modified AgI/TiO2 composites for photocatalytic degradation of p-chlorophenol under visible light irradiation. J Colloid Interface Sci 378:159–166
Sekizawa K, Maeda K, Domen K, Koike K, Ishitani O (2013) Artificial Z-scheme constructed with a supramolecular metal complex and semiconductor for the photocatalytic reduction of CO2. J Am Chem Soc 135:4596–4599
Qi H, Wolfe J, Fichou D, Chen Z (2016) Cu2O photocathode for low bias photoelectrochemical water splitting enabled by NiFe-layered double hydroxide co-catalyst. Sci Rep 6:30882
Zhang H, Fan X, Quan X, Chen S, Yu H (2011) Graphene sheets grafted Ag@AgCl hybrid with enhanced plasmonic photocatalytic activity under visible light. Environ Sci Technol 45:5731–5736
Xu Y, Xu H, Yan J, Li H, Huang L, Zhang Q, Huang C, Wan H (2013) A novel visible-light-response plasmonic photocatalyst CNT/Ag/AgBr and its photocatalytic properties. Phys Chem Chem Phys 15:5821–5830
Wang Y, Xia M, Li K, Shen X, Muhanmood T, Wang F (2016) Facile solvothermal synthesis of a high-efficiency CNNs/Ag/AgCl plasmonic photocatalyst. Phys Chem Chem Phys 18:27257–27264
Min Y, He G, Xu Q, Chen Y (2014) Self-assembled encapsulation of graphene oxide/Ag@AgCl as a Z-scheme photocatalytic system for pollutant removal. J Mater Chem A 2:1294–1301
Zhu M, Chen P, Liu M (2012) Highly efficient visible-light-driven plasmonic photocatalysts based on graphene oxide-hybridized one-dimensional Ag/AgCl heteroarchitectures. J Mater Chem 22:21487–21494
Zeng C, Guo M, Tian B, Zhang J (2013) Reduced graphene oxide modified Ag/AgBr with enhanced visible light photocatalytic activity for methyl orange degradation. Chem Phys Lett 575:81–85
Zhang D, Tang H, Wang Y, Wu K, Huang H, Tang G, Yang J (2014) Synthesis and characterization of graphene oxide modified AgBr nanocomposites with enhanced photocatalytic activity and stability under visible light. Appl Surf Sci 319:306–311
Reddy DA, Lee S, Choi J, Park S, Ma R, Yang H, Kim TK (2015) Green synthesis of AgI-reduced graphene oxide nanocomposites: Toward enhanced visible-light photocatalytic activity for organic dye removal. Appl Surf Sci 341:175–184
Shi H, Chen J, Li G, Nie X, Zhao H, Wong P-K, An T (2013) Synthesis and characterization of novel plasmonic Ag/AgX-CNTs (X = Cl, Br, I) nanocomposite photocatalysts and synergetic degradation of organic pollutant under visible light. ACS Appl Mater Interfaces 5:6959–6967
Xiao X, Zhang W, Yu J, Sun Y, Zhang Y, Dong F (2016) Mechanistic understanding of ternary Ag/AgCl@La(OH)3 nanorods as novel visible light plasmonic photocatalysts. Catal Sci Technol 6:5003–5010
Xu H, Xu Y, Li H, Xia J, Xiong J, Yin S, Huang C, Wan H (2012) Synthesis, characterization and photocatalytic property of AgBr/BiPO4 heterojunction photocatalyst. Dalton Trans 41:3387–3394
Peng T, Hu C, Hu X, Zhou X, Qu J (2012) Enhanced photodegradation of toxic pollutants on plasmonic Au–Ag–AgI/Al2O3 under visible irradiation. Catal Lett 142:646–654
Padervand M, Reza Elahifard M, Vatan Meidanshahi R, Ghasemi S, Haghighi S, Reza Gholami M (2012) Investigation of the antibacterial and photocatalytic properties of the zeolitic nanosized AgBr/TiO2 composites. Mater Sci Semicon Process 15:73–79
Lin H, Cao J, Luo B, Xu B, Chen S (2012) Synthesis of novel Z-scheme AgI/Ag/AgBr composite with enhanced visible light photocatalytic activity. Catal Commun 21:91–95
Li X, Yu J, Low J, Fang Y, Xiao J, Chen X (2015) Engineering heterogeneous semiconductors for solar water splitting. J Mater Chem A 3:2485–2534
Fan H, Zhu J, Sun J, Zhang S, Ai S (2013) Ag/AgBr/Co–Ni–NO3 layered double hydroxide nanocomposites with highly adsorptive and photocatalytic properties. Chem Eur J 19:2523–2530
Vinoth R, Karthik P, Muthamizhchelvan C, Neppolian B, Ashokkumar M (2016) Carrier separation and charge transport characteristics of reduced graphene oxide supported visible-light active photocatalysts. Phys Chem Chem Phys 18:5179–5191
Gao W, Ran C, Wang M, Li L, Sun Z, Yao X (2016) The role of reduction extent of graphene oxide in the photocatalytic performance of Ag/AgX (X = Cl, Br)/rGO composites and the pseudo-second-order kinetics reaction nature of the Ag/AgBr system. Phys Chem Chem Phys 18:18219–18226
Zhao G, Jiang L, He Y, Li J, Dong H, Wang X, Hu W (2011) Sulfonated graphene for persistent aromatic pollutant management. Adv Mater 23:3959–3963
Cai B, Lv X, Gan S, Zhou M, Ma W, Wu T, Li F, Han D, Niu L (2013) Advanced visible-light-driven photocatalyst upon the incorporation of sulfonated graphene. Nano 5:1910
Hu H, Zhao Z, Wan W, Gogotsi Y, Qiu J (2013) Ultralight and highly compressible graphene aerogels. Adv Mater 25:2219–2223
Reddy DA, Choi J, Lee S, Ma R, Kim TK (2015) Green synthesis of AgI nanoparticle-functionalized reduced graphene oxide aerogels with enhanced catalytic performance and facile recycling. RSC Adv 5:67394–67404
Chemseddine A, Boehm HP (1990) A study of the primary step in the photochemical degradation of acetic acid and chloroacetic acids on a TiO2 photocatalyst. J Mol Catal 60:295–311
D’Oliveira J-C, Minero C, Pelizzetti E, Pichat P (1993) Photodegradation of dichlorophenols and trichlorophenols in TiO2 aqueous suspensions: kinetic effects of the positions of the Cl atoms and identification of the intermediates. J Photochem Photobiol A Chem 72:261–267
Mills G, Hoffmann MR (1993) Photocatalytic degradation of pentachlorophenol on titanium dioxide particles: identification of intermediates and mechanism of reaction. Environ Sci Technol 27:1681–1689
Kormann C, Bahnemann DW, Hoffmann MR (1991) Photolysis of chloroform and other organic molecules in aqueous titanium dioxide suspensions. Environ Sci Technol 25:494–500
Carraway ER, Hoffman AJ, Hoffmann MR (1994) Photocatalytic oxidation of organic acids on quantum-sized semiconductor colloids. Environ Sci Technol 28:786–793
D’Oliveira JC, Al-Sayyed G, Pichat P (1990) Photodegradation of 2- and 3-chlorophenol in titanium dioxide aqueous suspensions. Environ Sci Technol 24:990–996
Hidaka H, Zhao J, Pelizzetti E, Serpone N (1992) Photodegradation of surfactants. 8. Comparison of photocatalytic processes between anionic DBS and cationic BDDAC on the titania surface. J Phys Chem 96:2226–2230
Wang P, Huang B, Qin X, Zhang X, Dai Y, Wei J, Whangbo M-H (2008) Ag@AgCl: A highly efficient and stable photocatalyst active under visible light. Angew Chem Int Ed 47:7931–7933
Ollis DF, Pelizzetti E, Serpone N (1991) Photocatalyzed destruction of water contaminants. Environ Sci Technol 25:1522–1529
Albert M, Gao YM, Toft D, Dwight K, Wold A (1992) Photoassisted gold deposition of titanium dioxide. Mater Res Bull 27:961–966
Dominguez A, Tardajos G, Aicart E, Perez-Casas S, Trejo LM, Costas M, Patterson D, van Tra H (1993) Van der Waals liquids, Flory theory and mixing functions for chlorobenzene with linear and branched alkanes. J Chem Soc Faraday Trans 89:89–93
Borgarello E, Serpone N, Emo G, Harris R, Pelizzetti E, Minero C (1986) Light-induced reduction of rhodium(III) and palladium(II) on titanium dioxide dispersions and the selective photochemical separation and recovery of gold(III), platinum(IV), and rhodium(III) in chloride media. Inorg Chem 25:4499–4503
Kudo A, Miseki Y (2009) Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev 38:253–278
Maeda K, Takata T, Hara M, Saito N, Inoue Y, Kobayashi H, Domen K (2005) GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J Am Chem Soc 127:8286–8287
Sato S, Morikawa T, Kajino T, Ishitani O (2013) A highly efficient mononuclear iridium complex photocatalyst for CO2 reduction under visible light. Angew Chem 125:1022–1026
Wang C, Xie Z, deKrafft KE, Lin W (2011) Doping metal–organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J Am Chem Soc 133:13445–13454
Pau MYM, Lipscomb JD, Solomon EI (2007) Substrate activation for O2 reactions by oxidized metal centers in biology. Proc Natl Acad Sci 104:18355–18362
Mallat T, Baiker A (2004) Oxidation of alcohols with molecular oxygen on solid catalysts. Chem Rev 104:3037–3058
Sawyer DT, Valentine JS (1981) How super is superoxide? Accounts Chem Res 14:393–400
Chen C, Ma W, Zhao J (2010) Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem Soc Rev 39:4206–4219
Zheng Z, Chen C, Bo A, Zavahir FS, Waclawik ER, Zhao J, Yang D, Zhu H (2014) Visible-light-induced selective photocatalytic oxidation of benzylamine into imine over supported Ag/AgI photocatalysts. ChemCatChem 6:1210–1214
Wang P, Tang Y, Dong Z, Chen Z, Lim T-T (2013) Ag-AgBr/TiO2/RGO nanocomposite for visible-light photocatalytic degradation of penicillin G. J Mater Chem A 1:4718–4727
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Ye L, Liu J, Gong C, Tian L, Peng T, Zan L (2012) Two different roles of metallic Ag on Ag/AgX/BiOX (X = Cl, Br) visible light photocatalysts: surface plasmon resonance and Z-scheme bridge. ACS Catal 2:1677–1683
Hu P, Cao Y, Jia D, Li Q, Liu R (2014) Engineering the metathesis and oxidation-reduction reaction in solid state at room temperature for nanosynthesis. Sci Rep 4:4153
Wang J, An C, Liu J, Xi G, Jiang W, Wang S, Zhang Q-H (2013) Graphene oxide coupled AgBr nanosheets: an efficient dual-functional visible-light-responsive nanophotocatalyst with enhanced performance. J Mater Chem A 1:2827–2832
Wang D, Guo L, Zhen Y, Yue L, Xue G, Fu F (2014) AgBr quantum dots decorated mesoporous Bi2WO6 architectures with enhanced photocatalytic activities for methylene blue. J Mater Chem A 2:11716–11727
Baetzold RC (1997) Calculated properties of Ag clusters on silver halide cubic surface sites. J Phys Chem B 101:8180–8190
Dong R, Tian B, Zeng C, Li T, Wang T, Zhang J (2013) Ecofriendly synthesis and photocatalytic activity of uniform cubic Ag@AgCl plasmonic photocatalyst. J Phys Chem C 117:213–220
An C, Wang R, Wang S, Zhang X (2011) Converting AgCl nanocubes to sunlight-driven plasmonic AgCl: Ag nanophotocatalyst with high activity and durability. J Mater Chem 21:11532–11536
Hu C, Peng T, Hu X, Nie Y, Zhou X, Qu J, He H (2010) Plasmon-induced photodegradation of toxic pollutants with Ag−AgI/Al2O3 under visible-light irradiation. J Am Chem Soc 132:857–862
Muskens OL, Del Fatti N, Vallée F (2006) Femtosecond response of a single metal nanoparticle. Nano Lett 6:552–556
Inouye H, Tanaka K, Tanahashi I, Hirao K (1998) Ultrafast dynamics of nonequilibrium electrons in a gold nanoparticle system. Phys Rev B 57:11334–11340
Tisdale WA, Williams KJ, Timp BA, Norris DJ, Aydil ES, Zhu X-Y (2010) Hot-electron transfer from semiconductor nanocrystals. Science 328:1543–1547
Kaelberer T, Fedotov VA, Papasimakis N, Tsai DP, Zheludev NI (2010) Toroidal dipolar response in a metamaterial. Science 330:1510–1512
Wang D, Li Y, Li Puma G, Wang C, Wang P, Zhang W, Wang Q (2013) Ag/AgCl@helical chiral TiO2 nanofibers as a visible-light driven plasmon photocatalyst. Chem Commun 49:10367–10369
Zhou X, Hu C, Hu X, Peng T (2012) Enhanced electron transfer and silver-releasing suppression in Ag–AgBr/titanium-doped Al2O3 suspensions with visible-light irradiation. J Hazard Mater 219–220:276–282
Zhou X, Hu C, Hu X, Peng T, Qu J (2010) Plasmon-assisted degradation of toxic pollutants with Ag−AgBr/Al2O3 under visible-light irradiation. J Phys Chem C 114:2746–2750
Seki K, Yanagi H, Kobayashi Y, Ohta T, Tani T (1994) UV photoemission study of dye/AgBr interfaces in relation to spectral sensitization. Phys Rev B 49:2760–2767
Jiang J, Li H, Zhang L (2012) New insight into daylight photocatalysis of AgBr@Ag: Synergistic effect between semiconductor photocatalysis and plasmonic photocatalysis. Chem Eur J 18:6360–6369
Ingram DB, Linic S (2011) Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: Evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J Am Chem Soc 133:5202–5205
Chang Y, Xu J, Zhang Y, Ma S, Xin L, Zhu L, Xu C (2009) Optical properties and photocatalytic performances of Pd modified ZnO samples. J Phys Chem C 113:18761–18767
Ravelli D, Dondi D, Fagnoni M, Albini A (2009) Photocatalysis. A multi-faceted concept for green chemistry. Chem Soc Rev 38:1999–2011
Bae E, Choi W (2003) Highly enhanced photoreductive degradation of perchlorinated compounds on dye-sensitized metal/TiO2 under visible light. Environ Sci Technol 37:147–152
Wu T, Liu G, Zhao J, Hidaka H, Serpone N (1998) Photoassisted degradation of dye pollutants. V. self-photosensitized oxidative transformation of Rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J Phys Chem B 102:5845–5851
Takeshita K, Sasaki Y, Kobashi M, Tanaka Y, Maeda S, Yamakata A, Ishibashi T-a, Onishi H (2004) Effect of annealing temperature on back electron transfer and distribution of deep trap sites in dye-sensitized TiO2, studied by time-resolved infrared spectroscopy. J Phys Chem B 108:2963–2969
Hagfeldt A, Graetzel M (1995) Light-induced redox reactions in nanocrystalline systems. Chem Rev 95:49–68
Moser JE, Grätzel M (1993) Observation of temperature independent heterogeneous electron transfer reactions in the inverted Marcus region. Chem Phys 176:493–500
Kuciauskas D, Freund MS, Gray HB, Winkler JR, Lewis NS (2001) Electron transfer dynamics in nanocrystalline titanium dioxide solar cells sensitized with ruthenium or osmium polypyridyl complexes. J Phys Chem B 105:392–403
Zhang X, Li J, Lu X, Tang C, Lu G (2012) Visible light induced CO2 reduction and Rh B decolorization over electrostatic-assembled AgBr/palygorskite. J Colloid Interface Sci 377:277–283
Glaus S, Calzaferri G (2003) The band structures of the silver halides AgF, AgCl, and AgBr: A comparative study. Photochem Photobiol Sci 2:398–401
Lou Z, Huang B, Ma X, Zhang X, Qin X, Wang Z, Dai Y, Liu Y (2012) A 3D AgCl hierarchical superstructure synthesized by a wet chemical oxidation method. Chem Eur J 18:16090–16096
Hou Y, Zuo F, Ma Q, Wang C, Bartels L, Feng P (2012) Ag3PO4 oxygen evolution photocatalyst employing synergistic action of Ag/AgBr nanoparticles and graphene sheets. J Phys Chem C 116:20132–20139
Schürch D, Currao A, Sarkar S, Hodes G, Calzaferri G (2002) The silver chloride photoanode in photoelectrochemical water splitting. J Phys Chem B 106:12764–12775
Funding
This work was supported by NSFC, China (21622509, 21527806 and 21475122), Department of Science and Techniques of Jilin Province (20150201001GX and 20150203002YY), Jilin Province Development and Reform Commission (2016C014, 2017C053-1), Science and Technology Bureau of Changchun (15SS05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, Y., Han, D., Song, Z. et al. Regulations of silver halide nanostructure and composites on photocatalysis. Adv Compos Hybrid Mater 1, 269–299 (2018). https://doi.org/10.1007/s42114-017-0005-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-017-0005-2