Abstract
The novel SARS-CoV-2 has spread to virtually all countries of the world infecting millions of people, the medical burden of this disease obviously being enormous. The gonads of both sexes are among the organs that may be affected by COVID-19 and/or may affect the severity of the disease. The clinical spectrum of SARS-CoV-2 infection clearly differs between genders. The current evidence indicates that the underlying mechanism of such an interaction could be associated with genetic, hormonal, and immunological differences, as well as with gender differences in such habits as smoking and alcohol use. On the other hand, there are controversies as to how and to what extent the gonads could be affected by COVID-19, possibly impacting upon sex steroids, fertility, and other functions. This review underlines the possible mechanisms that could clarify these questions concerning COVID-19 and the gonads. In addition, reference is made to potential new treatment modalities presently under investigation, these supported by accumulating data published in the recent literature.
Similar content being viewed by others
Introduction
The novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has affected millions of people in virtually every country of the world, while ever greater awareness of the medical burden of the disease and knowledge about its management and treatment are constantly developing. Coronavirus disease 2019 (COVID-19) is a disease of multiorgan involvement, but emerging data point to the possible effects on organs and systems that have not until now been fully considered. The gonads of both males and females are among suspected organs which may be affected by and/or affect the severity of COVID-19. The SARS-CoV-2 pandemic may be called a sexually dimorphic disease, early Chinese reports [1, 2] and later global studies [3, 4] having suggested that women are less susceptible to COVID-19, and female patients exhibit a significantly lower mortality rate compared with males. There are a number of postulations regarding possible mechanisms driving this gender imbalance [5,6,7].
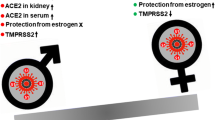
Angiotensin-converting enzyme 2 (ACE2) plays a key role in SARS-CoV-2 infection. It mediates viral infection but also exerts important regulatory effects on the immune system mainly during acute lung injury [8, 9]. The presence of ACE2 on almost all testicular cells, as documented in the literature [10, 11], raises important questions, among them whether male fertility and gonad functions are affected by SARS-CoV-2 infection. Meanwhile, it has also been shown that estrogen (E2) regulates the expression of SARS-CoV-2 receptor ACE2 in pulmonary epithelial cells [7]. Such emerging literature data also warrant investigation as to the pros and cons of estrogen therapy in COVID-19.
Estrogen and testosterone have different effects on the strength of immune responses, which may result in different disease courses and outcomes between males and females, with females exhibiting stronger immune responses than males [5, 12], this possibly being a reason for the better outcomes of females infected by COVID-19. The aim of this review is to clarify a number of issues regarding COVID-19 and the gonads of both sexes with the help of the emerging data published in the recent literature. We evaluate herein all studies on male and female reproductive functions associated with COVID-19 infection published in the English literature. A literature search was conducted in the PubMed, Scopus, Web of Science, ScienceDirect, Embase, and Google Scholar databases with search terms including “SARS-CoV-2,” “COVID-19,” “angiotensin-converting enzyme 2,” “transmembrane serine protease 2,” “androgen,” “estrogen,” “gonad,” “fertility,” “spermatogenesis,” “testosterone,” “male hypogonadism,” and “gender” until November, 2020. A total of 212 articles were found, of which 114 were included based on their relevance to this review and scope. Additional sources were identified from citations of the retrieved literature.
COVID-19 and the female gonads
Estrogen and ACE2
ACE2 is a transmembrane protein which mainly has protease functions, leading to cleavage of angiotensin I into inactive angiotensin [8, 13], thus counterbalancing the effects of the renin-angiotensin-aldosterone system (RAAS) [8, 14]. ACE2 additionally acts as a receptor for SARS-CoV-2 with high affinity and mediates the viral infection of cells, enabling easier human-to-human transmission [8, 15]. Transmembrane serine protease 2 (TMPRSS2) is also needed to allow fusion of the virus and host cell membranes [16].
ACE2 is mainly expressed in the human heart, kidney, lungs, liver, intestine, and testis [17]. More recently, it was found that ACE2 is also expressed in human ovaries, oocytes, and endometrial tissue, although its functional role is still unclear [18,19,20]. Emerging studies in the last decade have suggested that ACE2 may regulate the production of estradiol and progesterone [21] and may enhance ovulation [22, 23] and the maturation of human oocytes [24]. Furthermore, ACE-2 is thought to have an effect on menstrual cycles by regulating the regeneration of endometrium and myometrium activity [20]. TMPRSS2 is more broadly expressed than is ACE2, and co-expression of ACE2 and TMPRSS2 has been shown in testicular, endometrial, and placental cells and in nonhuman primate ovarian cells in various studies [25,26,27]. The latter strongly suggests that endometrial tissue, oocytes, and ovaries are possible candidate sites for the SARS-CoV-2 infection. However, there have to date been no reports of the virus in the female reproductive tract. In addition, current data on other coronaviruses, especially SARS-CoV-1, indicate that the female reproductive system may be spared from viral infection [28].
On the other hand, unlike ACE2 actions, E2 might affect ACE2 expression. Kimberly et al. observed that high concentrations of E2-treatment reduced ACE2 mRNA expression in differentiated airway epithelial cells. Although they conclude that this reduction might not necessarily translate into a reduction of ACE2 protein on the cell surface, E2 can regulate the expression of ACE2 in differentiated airway epithelial cells [7].
Estrogen and sexual dimorphism in COVID-19
All reports, including the most recent, confirm that female patients exhibit a lower disease morbidity and mortality rate compared to male patients after COVID-19 infection [29, 30]. An Italian retrospective data reported that among 1591 consecutive severe COVID-19 patients referred for admission to intensive care, 1304 (82%) were men, this being similar for all age groups [31]. Moreover, large studies showed that the male-to-female mortality ratio was 2, particularly among patients over 70 years of age [26]. The question which is thus posed is why women are protected. In this regard, recent studies have postulated a number of mechanisms to account for this phenomenon, including ACE2 expression, smoking, social role, and presence of comorbidities [32, 33]. However, the immunomodulatory effects of sex hormones seem to be the most important factor explaining the lower mortality rates among women [5, 26, 34].
Estrogen is the main female sex hormone. To date, many different studies have shown that E2 regulates the strength of both innate and adaptive immunity [5, 12, 35,36,37]. E2 exerts its effects through estrogen receptors (ERs), which are expressed differently in the subsets of immune cells: ERα is highly expressed on T cells and ERβ on B cells [38]. During the innate immune response, ERs on monocytes, macrophages, and neutrophils are activated via estrogen, leading to release of proinflammatory cytokines, chemokine, and interferon [36]. Further activation of lymphocytes and alveolar macrophages leads to decreasing virus replication, resulting in a rapid defensive response [36, 37]. After this non-specific first barrier targeting invaders and slowing infection, adaptive immunity comes into play. Estrogen stimulates the humoral response to viral infections by inducing higher levels of antibodies and activating antibody-producing cells. This elucidates why females have stronger cell-dependent and humoral responses to infection and also to vaccination than do males [39]. Additionally, it was confirmed that leukocyte function and macrophage phagocytosis are more efficient in females than are these processes in males; thus, pathogen elimination is more rapid in females [40].
However, regarding the immunomodulatory effects of estrogen, the immune response to pathological conditions other than infection is different, especially with respect to autoimmunity [26]. Female sex is disadvantageous for autoimmunity, estrogen facilitating the development of immune-pathogenic effects. E2 acts through its peripheral metabolites in autoimmune rheumatic diseases, and the intracrine synthesis of active estrogen metabolites at the level of cells involved in the immune response seems to be the common pathway for immunostimulatory activity [41].
COVID-19 and female reproduction
Since SARS-CoV-2 is a novel virus, data regarding its impact on human reproduction are as yet limited. To date, there have been no reports of the virus in the female reproductive tract, in vaginal secretions, in amniotic fluid, or in peritoneal fluid [25]. Although there is abundant expression of ACE2 in the ovaries and oocytes, no information at present exists as to possible ovarian dysfunction after COVID-19 infection. The evidence thus far therefore suggests that SARS-CoV-2 infection is unlikely to have long-term effects on female fertility [25, 27].
ACE2 is widely expressed in the human placenta and the umbilical cord [42]. Therefore, the potential for vertical transmission of SARS-CoV-2 and a consequent impact on early and late pregnancy outcomes is another area of uncertainty that needs to be explored. Most of the literature on pregnancy in patients with COVID-19 has covered women in labor or near term or else during the third trimester. In a large cohort of 3923 women admitted for delivery, only 0.43% of cases were SARS-CoV-2-positive, and most of them were asymptomatic; on the other hand, no neonates were positive for SARS-CoV-2 at 24 h of life [43]. However, studies conducted in pregnant women with COVID-19 report little increase in maternal morbidity, especially as concerns the respiratory system and preterm birth [44]. Nevertheless, pregnancy outcomes of women with severe COVID-19 infection do not appear to greatly differ from those of non-infected mothers [25, 45, 46]. Limited data concerning early pregnancy showed that miscarriage was rare [44, 45]. Most cases were asymptomatic and the pregnancy course did not differ from what was expected [47].
Vertical transmission is another concern. Although expression of ACE2 has been documented in the placenta, co-expression with TMPRSS2 has not been reported, suggesting that placental cells may act as a barrier to vertical SARS-CoV-2 transmission [27]. This is compatible with the current studies on newborns born to SARS-CoV-2 infected mothers. Most of the early clinical evidence suggests that vertical transmission of SARS-CoV-2 from mother to newborn does not occur [27, 45, 47].
Estrogen as a treatment for COVID-19
The immunomodulatory effects of estrogen are thought to be related to its levels. E2 fluctuations during menstruation are associated with differences in immune activities; this is also reported in patients using exogenous estrogen for contraception or hormone replacement therapy [34, 48]. In vitro studies have demonstrated that high concentrations of E2 treatment may reduce ACE2 mRNA expression, which suggests that immunomodulation is more robust with high levels [7]. On the other hand, it is known that estrogen has a protective role in endothelial function [49] by preventing or reducing lung and intestinal injuries after ischemic trauma [50, 51]. E2 also has anti-inflammatory effects similar to those of glucocorticoids [52]. In addition, estrogen exerts antiviral activity by increasing virus-specific CD8 cells, decreasing the transcription of virus genes and virus trafficking [5]. Emerging evidence from these experimental studies suggests that estrogens can modulate lung inflammation; furthermore, they may be effective in the prevention and treatment of COVID-19 [5, 26, 53, 54]. New trials with estrogen treatment for COVID-19 are ongoing [55]. Table 1 summarizes the evidence of a relationship between COVID-19 and the female gonads.
COVID-19 and the male gonadal axis
Factors determining higher COVID-19 prevalence and mortality in men
Sexual dimorphism and comorbid diseases
COVID-19 disease primarily affects vulnerable individuals with preexisting conditions, among whom it has caused more than 600,000 deaths [2, 56]. Although advanced age is the main determinant of the severity of COVID-19 disease course and related mortality in both sexes, men have worse clinical outcomes independent of age [3]. It is reported that worse results are seen in men at all stages of the disease, such as admission to intensive care units and need for ventilation support [4]. Sexual dimorphism in prevalence and severity of numerous diseases is not limited to viral diseases, also being observed in cardiovascular, lung, and autoimmune diseases. Generally speaking, unhealthy lifestyle habits such as smoking and alcohol consumption, often leading to cardiovascular diseases, hypertension, and diabetes, are more common in men, and all of these occur at younger ages than in women [57, 58]. It has moreover been determined that these comorbidities are the most common conditions related to increased COVID-19 mortality. In many studies, hypertension was the most common comorbidity, followed by diabetes and coronary heart disease [59]. Studies have demonstrated similar sexual differences during past coronavirus infections such as SARS-CoV-1 and MERS-CoV [60]. In a study by Channappanavar et al., female and male mice of different age groups were analyzed for their susceptibility to infection by infection with SARS-CoV-1. They observed that male mice were more susceptible to SARS-CoV-1 than female mice, and the significance was more prominent with advancing age [36]. Sexual selectivity has attracted considerable attention, and male gender has been shown to be the most important independent risk factor for COVID-19 in a study from China. This trend was still obvious when comorbidities were excluded [61, 62].
Androgen levels
Although androgen levels were proposed as a critical factor in determining male susceptibility to COVID-19, the effect of androgen levels has not, in fact, been demonstrated in many research studies. While males show similarly low testosterone levels both before puberty and at advanced ages, boys are less susceptible to COVID-19 infection than older male adults. In contrast, despite the high testosterone levels of male neonates and post-pubertal boys, the COVID-19 infection rate was not increased in babies at the 6th to 8th week after delivery [29], while COVID-19-related mortality is not particularly high among adult men, who display the highest testosterone levels among adult males [63, 64]. All these results indicate that age and comorbid diseases are more important factors than testosterone levels in the spread and severity of COVID-19 disease among men [65]. A large German cohort study reported that the majority of the male patients with COVID-19 who were transferred to intensive care units have had low testosterone levels [66]. Rastrelli et al. also reported that poor prognosis and high mortality rates were associated with lower total and free testosterone levels in SARS-CoV-2 infected men when they were admitted to respiratory intensive care units [67]. To conclude, according to emerging data, gender disparity in COVID-19 disease severity cannot be explained by androgen levels alone.
Could the use of cigarettes affect the frequency and severity of the disease?
Unhealthy habits like smoking and alcohol consumption are more common in men than in women. Initially, these habits were thought to be possible determinants of sexual disparity in COVID-19 disease severity: some of the studies indicated that cigarette smoking induces elevated ACE2 expression in the respiratory tract and that smokers have a higher susceptibility to COVID-19 than nonsmokers [57, 68, 69]. Of interest, the high risk of smoking during disease activity was also demonstrated during previous MERS-CoV infections [70]. In contrast, however, a study from Italy pointed out that 84.4% of patients with COVID-19-related pneumonia had never smoked and 20 (15.2%) were former smokers [71]. Another study from New York City and a meta-analysis written by Lippi et al. have also reported that current smoking rates among COVID-19 patients were below those of their respective general populations [72, 73]. Nonetheless, although no direct relationship can be demonstrated between current smoking and COVID-19 disease activity, SARS-CoV-2 infection is seen to be more severe in smokers [74].
Androgen receptors, CAG repeats, and androgen sensitivity
Different theories have been proposed to explain sexual dimorphism in COVID-19, such as variability in androgen sensitivity, androgen receptor action, and even gene polymorphism [75]. However, there are contradictions among many of the proposed ideas. Androgenetic alopecia (AGA), also known as male pattern hair loss, may be a symptom of hyperandrogenism. This was first observed in Spain where there were very high rates of patient mortality: the finding was named the Gabrin sign. Goren et al. noted the presence of the bilateral pneumonia accompanied by AGA in 71% of men diagnosed with COVID-19 [75]. It is well known that androgen sensitivity is associated with CAG repeat length and polymorphisms on the androgen receptor gene. Male sex steroids exert their effects through the androgen receptor in both men and women. Hyperandrogenic findings such as oily skin, acne, and androgenetic alopecia have been shown to be associated with short CAG repeat length [61]. Interestingly, short CAG repeats cause different metabolic consequences in males and females. While high androgen levels in men are associated with favourable health status, such as increased insulin sensitivity and lean body mass, they can lead to abdominal obesity and insulin resistance in women [76, 77]. Similarly, in women diagnosed with PCOS, it is believed that shorter CAG repeats in the androgen receptor gene may cause negative effects [78]. Therefore, it is thought that worse outcomes of COVID-19 in men may be associated with CAG repeat length on the androgen receptor gene.
Androgens and immune modulation
As demonstrated in previous reports, immune response might be modulated by androgens, and testosterone can suppress innate immune responses. Ultimately, a less robust immune response is generated in males than in women, as well as a decrease in antibody response to viral infection [58, 79]. It has been hypothesized that this is one of the possible mechanisms that may weaken immune defense against SARS-CoV-2 [57]. The effect of androgens on immune modulation is not limited only to antibody response: it can also increase the number and function of circulating neutrophils. In this way, androgens can increase the production of growth factors and interleukins such as IL-1b, IL-10, and IL-2. It is hypothesized that this could be one of the possible mechanisms that may alter antiviral immune response to SARS-CoV-2 [80].
Viral entry facilitated by androgens and effects of X chromosomes
Endocytosis and fusion of the viral membrane with the membrane of the target cell have been defined as entryways of SARS-CoV-2 to the target cell. The main target cell of such viruses is type II pneumocystis, while ACE2 and TMPRSS2 enable the entry of SARS-CoV-2 into the airways [81]. ACE2 plays a role as a receptor protein, while TMPRSS2 functions as a protease that cleaves the viral spike protein. Therefore, both ACE2 and TMPRSS2, regulated by the androgen receptor, are essential for viral activity and hosting of SARS-CoV-2 in pneumocystis [82, 83]. Gene expression of TMPRSS2 and ACE has been observed in many tissues, such as the pancreas, kidney, lungs, and both male and female gonads. The TMPRSS2 gene encodes TMPRSS2 as an enzyme, and it has been reported that expression of this gene was upregulated by androgenic hormones mainly in prostate cancer cells. Additional studies also showed that ACE2 and TMPRSS2 gene expression can be increased in all tissues by androgens [81, 84,85,86]. Both androgen receptor and ACE2 genes, and most of the genes regulating immune responses are located on the X chromosome. Some of these genes may play a role as disease-susceptibility genes in both sexes. The fact that males have only one copy of the X chromosome may play a part in male vulnerability during severe viral infections. Therefore, androgen sensitivity and production of the sex-specific steroids may be important factors for disease severity in men who are more susceptible to these effects [36, 87].
Androgens and lungs
The effect of androgens on the pulmonary system might also explain why males are more vulnerable to viral attacks. Viral infections may show opposite effects between aged males and pre-pubertal children [88, 89]. In a study by Channappanavar et al., male mice infected with SARS-CoV-2 displayed higher virus titers and increased inflammatory cell infiltration in their lungs. In addition, it was noted that male mice had increased pulmonary vascular permeability and alveolar edema in their lungs compared to age-matched infected female mice [36]. It is well known from other mouse studies that male neonates are more prone to developing neonatal respiratory distress syndrome than female. Moreover, during fetal development, the lungs of male fetuses develop more slowly, while they have a smaller number of types II pneumocystis and a smaller amount of surfactant, which plays an important protective role for the lungs and facilitates respiration. Surfactant synthesis is influenced by the androgen receptor. In addition, androgen receptor gene expression has been shown throughout the respiratory tract predominantly in the bronchial epithelium and PTII cells [90, 91]. These findings show that male selectivity of the lung pathologies might start from the mother’s womb and is multifactorial.
Effects of COVID-19 on the hypothalamic-pituitary-testicular and male reproductive axes
The hypothalamic-pituitary-testicular (HPT) axis is primarily responsible for regulating reproductive activity and orchestrating the release of both centrally and peripherally produced sex hormones. This is a very dynamic axis, and its activity lasts a lifetime; however, it may easily be altered by external factors. The hypothalamic-pituitary-adrenal, thyroid, and gonad axes can all be rapidly affected by viral infections such as COVID-19 [92, 93]. This effect can be exerted either directly via viral infiltration or indirectly as a consequence of systemic responses. During severe infection caused by SARS-CoV-2, many factors might affect this axis, such as the virus itself, medications used for treatment, environmental disinfectants, and psychological effects of the disease. Essentially, both the brain and the testicles are well protected against external influences via the mechanism called the blood-brain and blood-testicular barrier, respectively [94, 95]. Some of the viruses can pass these barriers directly or owing to damaged barriers caused by the effects of systemic or local inflammation.
In the Endocrine Society Guidelines, hypogonadism is defined as “failure of the testes to produce physiological concentrations of testosterone and/or a normal number of spermatozoa due to pathology at one or more levels of the HPT axis.” Hypogonadism may be primary, due to testicular dysfunction, or secondary, due to the hypothalamic/pituitary diseases, and in some instances, the two may occur together. The cause of hypogonadism may be organic, or else, it can be due to congenital, structural, or destructive pathology that results in permanent hypogonadism, or functional caused by potentially reversible conditions that suppress gonadotropin and T concentrations [96]. All types of hypogonadism described above can be seen during SARS-Cov-2 infection. Concerning the hypogonadism patient with COVID-19, he or she may be affected by a wide spectrum of the disease [96,97,98].
It has been shown that ACE2, which is the primary entry route of SARS-CoV-1 to the target cells, is also expressed in hypothalamic and pituitary tissues [99], though to date, there are few data showing that the virus directly affects these tissues. In an autopsy series of patients who had died due to SARS-CoV-1, viral genomes have been identified in hypothalamic tissues, along with infarct, edema, and neuronal degeneration [100]. Many of the patients had described neurologic symptoms, anosmia, and ageusia during COVID-19 infection, symptoms that may be linked to a central cause and reflect the role of the hypothalamus and related organs [101]. Compared with to healthy controls, in previous SARS virus attacks, serum prolactin, FSH, and LH levels were increased, and estradiol and progesterone levels were decreased, reflecting primary hypogonadism. By contrast, in cases of SARS-CoV-1, secondary hypothyroidism and adrenal insufficiency have frequently been observed, these possibly reflecing hypophysitis or direct hypothalamic involvements [92]. However, there have been conflicting findings during the current SARS-CoV-2 infection. In men with COVID-19, Ma et al. reported high levels of PRL and LH vs. low testosterone and follicle-stimulating hormone levels, reflecting primary testicular damage during active disease [102]. It is well known that testosterone levels in patients with COVID-19 are not a reliable marker of testicular function, as acute and severe infection can suppress the HPT axis and decrease circulating testosterone [96].
Almost all the testicular cells including seminiferous duct cells, spermatogonia, Leydig cells, and Sertoli cells express very high levels of ACE2 mRNA [103, 104]. This causes all tissues of the testicle to be affected by COVID 19 infection. As a result, the disease can easily spread to all testicular tissue and may affect male reproductive function. ACE2 expression on adult Leydig cells may play a crucial role in regulating steroidogenesis, whereas on Sertoli cells, it could affect sperm production. Orchitis, which can lead to germ cell apoptosis and disruption in spermatogenesis, was documented during the outbreak of SARS-CoV-1 in 2002 [105]. Additionally, orchitis has been revealed in testis autopsy specimens obtained from six patients who died of SARS-CoV-1 [106]. Not only SARS-CoV-1 but also many viruses such as HIV, hepatitis B and C, mumps, Epstein-Barr, and papilloma virus have been reported to affect the testicles. Although the testicles have a highly selective blood-testis barrier, it is well known that the male reproductive system is vulnerable to many viral infections [107, 108]. Holtmann et al. reported that, after a mean of 43 days following a positive SARS-CoV-2 result, the virus was not detected in the semen of recovered or acutely infected men with SARS-CoV-2 [109]. In contrast, about 15% of the semen samples from COVID-19 patients were reported positive for SARS-CoV-2 in another study. There are conflicting reports about the presence of SARS-CoV-2 in the semen of patients who were previously diagnosed with COVID-19. On the other hand, sexual transmission of the virus has not been shown to date [110,111,112,113].
Very recently, a study published by Li et al. demonstrated that histopathological testis and epididymal tissues were affected by COVID-19. Researchers have shown that the involvement is not limited to the testicular parenchyma but is also associated with increased apoptosis in germ cell lines. They detected a high ratio of CD3+ and CD68+ in interstitial cells, supporting the increased immune response against testicular tissue. In addition to decreased sperm concentration, increased seminal levels of IL-6, TNF-α, and MCP-1 were observed. These results showed that COVID-19 disease may have a greater impact on male fertility than expected [114]. Factors associating the higher prevalence and mortality in males and effects of SARS-CoV-2 on the male reproductive system are summarized in Table 2.
Conclusion
SARS-CoV-2 needs ACE2 and TMPRSS2 expression for viral activity, while androgens increase ACE2 and TMPRSS2 gene expression regardless of the levels. By contrast, estrogen might reduce ACE2 mRNA expression in differentiated airway epithelial cells. Therefore, viral entry seems to be easier in males, which may explain the gender difference in susceptibility to COVID-19. Females have stronger cell-dependent and humoral responses to infection, and pathogen elimination is more rapid in females than in males. Immunomodulatory effects of sex hormones may explain the sexual difference in COVID-19 disease severity and mortality. On the other hand, data concerning the effect of SARS-CoV-2 on the gonads, especially in males, are increasing. Both reproduction and sex steroid synthesis of males are found to be diminished by SARS-CoV-2 infection in some studies. Therefore, follow-up of patients to monitor for gonad function and even fertility should be encouraged after COVID-19. Lastly, sex steroids, mainly estrogen, can modulate inflammation and may therefore be effective in the prevention and treatment of COVID-19. Even androgen-modulating drugs could be evaluated as a potential treatment for COVID-19.
References
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. https://doi.org/10.1001/jama.2020.2648
Galbadage T, Peterson BM, Awada J et al (2020) Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes. Front Med (Lausanne) 7:348. https://doi.org/10.3389/fmed.2020.00348
Grasselli G, Greco M, Zanella A et al (2020) Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.3539
Breithaupt-Faloppa AC, Correia CJ, Prado CM, Stilhano RS, Ureshino RP, Moreira LFP (2020) 17β-estradiol, a potential ally to alleviate SARS-CoV-2 infection. Clinics (Sao Paulo) 75:e1980. https://doi.org/10.6061/clinics/2020/e1980
De Cauwer H (2020) The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies: comment. Intern Emerg Med:1–2. https://doi.org/10.1007/s11739-020-02406-z
Stelzig KE, Canepa-Escaro F, Schiliro M, Berdnikovs S, Prakash YS, Chiarella SE (2020) Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 318(6):L1280–L1281. https://doi.org/10.1152/ajplung.00153.2020
Liu MY, Zheng B, Zhang Y, Li JP (2020) Role and mechanism of angiotensin-converting enzyme 2 in acute lung injury in coronavirus disease 2019. Chronic Dis Transl Med 6(2):98–105. https://doi.org/10.1016/j.cdtm.2020.05.003
Lu R, Zhao X, Li J, et al (2020) Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 395(10224):565–574. https://doi.org/10.1016/S0140-6736(20)30251-8
Vishvkarma R, Rajender S (2020) Could SARS-CoV-2 affect male fertility? [published online ahead of print, 2020 Jun 23]. Andrologia:e13712. https://doi.org/10.1111/and.13712
Douglas GC, O'Bryan MK, Hedger MP, et al (2004) The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 145(10):4703–4711. https://doi.org/10.1210/en.2004-0443
Roved J, Westerdahl H, Hasselquist D (2017) Sex differences in immune responses:hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav 88:95–105. https://doi.org/10.1016/j.yhbeh.2016.11.017
Donoghue M, Hsieh F, Baronas E et al (2000) A novel angiotensin-converting enzymerelated carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87(5):E1–E9. https://doi.org/10.1161/01.res.87.5.e1
Albini A, Di Guardo G, Noonan DM, Lombardo M (2020) The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies [published online ahead of print, 2020 May 19]. Intern Emerg Med:1–8. https://doi.org/10.1007/s11739-020-02364-6
Wrapp D, Wang N, Corbett KS, et al (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 367(6483):1260–1263. https://doi.org/10.1126/science.abb2507
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q (2020) Structural basis for the recognition of SARSCoV-2 by full-length human ACE2. Science 367(6485):1444–1448. https://doi.org/10.1126/science.abb2762
Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275(43):33238–33243. https://doi.org/10.1074/jbc.M002615200
Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. (2011) Angiotensin-(1-7), its receptor mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril 95(1):176–181. https://doi.org/10.1016/j.fertnstert.2010.06.060
Qin S, Zhou YJ, Liu Y et al (2013) Expression and significance of ACE2-Ang-(1-7)-mas axis in the endometrium of patients with polycystic ovary syndrome. Zhonghua Yi Xue Za Zhi 93(25):1989–1992
Jing Y, Run-Qian L, Hao-Ran W et al (2020) Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod 26(6):367–373. https://doi.org/10.1093/molehr/gaaa030
Costa AP, Fagundes-Moura CR, Pereira VM, Silva LF, Vieira MA, Santos RA, Dos Reis AM. (2003) Angiotensin-(1-7): a novel peptide in the ovary. Endocrinology 144:1942–1948
Tonellotto dos Santos J, Ferreira R, Gasperin BG, Siqueira LC, de Oliveira JF, Santos RA, Reis AM, Goncalves PB (2012) Molecular characterization and regulation of the angiotensin-converting enzyme type2/angiotensin-(1-7)/MAS receptor axis during the ovulation process in cattle. J Renin-Angiotensin-Aldosterone Syst 13:91–98
Viana GE, Pereira VM, Honorato-Sampaio K, Oliveira CA, Santos RA, Reis AM (2011) Angiotensin-(1-7) induces ovulation and steroidogenesis in perfused rabbit ovaries. Exp Physiol 96:957–965
Cavallo IK, Dela Cruz C, Oliveira ML, Del Puerto HL, Dias JA, Lobach VN et al (2017) Angiotensin-(1-7) in human follicular fluid correlates with oocyte maturation. Hum Reprod 32:1318–1324
Segars J, Katler Q, McQueen DB et al (2020) Prior and novel coronaviruses, coronavirus disease 2019 (COVID-19), and human reproduction: what is known? Fertil Steril 113(6):1140–1149. https://doi.org/10.1016/j.fertnstert.2020.04.025
Cutolo M, Smith V, Paolino S (2020) Understanding immune effects of oestrogens to explain the reduced morbidity and mortality in female versus male COVID-19 patients. Comparisons with autoimmunity and vaccination. Clin Exp Rheumatol 38(3):383–386
Stanley KE, Thomas E, Leaver M, Wells D (2020) Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril 114(1):33–43. https://doi.org/10.1016/j.fertnstert.2020.05.001
Ding Y, He L, Zhang Q, et al (2004) Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 203(2):622–630. https://doi.org/10.1002/path.1560
Guan WJ, Ni ZY, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720. https://doi.org/10.1056/NEJMoa2002032
Adams JG, Walls RM (2020) Supporting the health care workforce during the COVID-19 global epidemic [published online ahead of print, 2020 Mar 12]. JAMA. https://doi.org/10.1001/jama.2020.3972
Grasselli G, Zangrillo A, Zanella A et al (2020) Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy [published online ahead of print, 2020 Apr 6]. JAMA 323(16):1574–1581. https://doi.org/10.1001/jama.2020.5394
Liu J, Ji H, Zheng W et al (2010) Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ 1(1):6. Published 2010 Nov 5. https://doi.org/10.1186/2042-6410-1-6
Antonello RM, Dal Bo E, De Cristofaro P, Luzzati R, Di Bella S (2020) The seXY side of COVID-19: what is behind female protection? Infez Med 28(2):288–289
Grandi G, Facchinetti F, Bitzer J (2020) The gendered impact of coronavirus disease (COVID-19): do estrogens play a role? Eur J Contracept Reprod Health Care 25(3):233–234. https://doi.org/10.1080/13625187.2020.1766017
Hughes GC, Choubey D (2014) Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol 10(12):740–751. https://doi.org/10.1038/nrrheum.2014.144
Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S (2017) Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol 198(10):4046–4053. https://doi.org/10.4049/jimmunol.1601896
Kovats S (2015) Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294(2):63–69. https://doi.org/10.1016/j.cellimm.2015.01.018
Khan D, Ansar Ahmed S (2016) The immune system is a natural target for estrogen action:opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol 6:635. Published 2016 Jan 6. https://doi.org/10.3389/fimmu.2015.00635
Lü FX, Abel K, Ma Z, et al. (2002). The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 128(1):10–20. https://doi.org/10.1046/j.1365-2249.2002.01780.x
Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW (2011) Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 118(22):5918–5927. https://doi.org/10.1182/blood-2011-03-340281
Cutolo M, Sulli A, Straub RH (2012) Estrogen metabolism and autoimmunity. Autoimmun Rev 11(6–7):A460–A464. https://doi.org/10.1016/j.autrev.2011.11.014
Valdés G, Neves LA, Anton L, et al (2006) Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 27(2–3):200–207. https://doi.org/10.1016/j.placenta.2005.02.015
Fassett MJ, Lurvey LD, Yasumura L et al (2020) Universal SARS-Cov-2 screening in women admitted for delivery in a large managed care organization [published online ahead of print, 2020 Jul 3]. Am J Perinatol. https://doi.org/10.1055/s-0040-1714060
Sentilhes L, De Marcillac F, Jouffrieau C et al (2020) COVID-19 in pregnancy was associated with maternal morbidity and preterm birth [published online ahead of print, 2020 Jun 15]. Am J Obstet Gynecol. S0002-9378(20)30639-6. https://doi.org/10.1016/j.ajog.2020.06.022
Ovalı F (2020) SARS-CoV-2 infection and the newborn. Front Pediatr 8:–294. Published 2020 May 22. https://doi.org/10.3389/fped.2020.00294
Cosma S, Borella F, Carosso A et al (2020) The "scar" of a pandemic: cumulative incidence of COVID-19 during the first trimester of pregnancy [published online ahead of print, 2020 Jul 7]. J Med Virol. https://doi.org/10.1002/jmv.26267
Thomas P, Alexander PE, Ahmed U et al (2020) Vertical transmission risk of SARS-CoV-2 infection in the third trimester: a systematic scoping review [published online ahead of print, 2020 Jul 1]. J Matern Fetal Neonatal Med:1–8. https://doi.org/10.1080/14767058.2020.1786055
Kaushic C, Roth KL, Anipindi V, Xiu F (2011) Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. J Reprod Immunol 88(2):204–209. https://doi.org/10.1016/j.jri.2010.12.004
Ma XL, Gao F, Chen J, et al. (2001) Endothelial protective and antishock effects of a selective estrogen receptor modulator in rats. Am J Physiol Heart Circ Physiol. 280(2):H876-H884. https://doi.org/10.1152/ajpheart.2001.280.2.H876
Breithaupt-Faloppa AC, Thais Fantozzi E, Romero DC et al (2014) Acute effects of estradiol on lung inflammation due to intestinal ischemic insult in male rats. Shock 41(3):208–213. https://doi.org/10.1097/SHK.0000000000000092
Ricardo-da-Silva FY, Fantozzi ET, Rodrigues-Garbin S et al (2017) Estradiol modulates local gut injury induced by intestinal ischemia-reperfusion in male rats. Shock 48(4):477–483. https://doi.org/10.1097/SHK.0000000000000873
Nadkarni S, McArthur S (2013) Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol 13(4):576–581. https://doi.org/10.1016/j.coph.2013.05.007
Suba Z (2020) Prevention and therapy of COVID-19 via exogenous estrogen treatment for both male and female patients. J Pharm Pharm Sci 23(1):75–85. https://doi.org/10.18433/jpps31069
Chen KH, Wang SF, Wang SY et al (2020) Pharmacological development of the potential adjuvant therapeutic agents against coronavirus disease 2019 [published online ahead of print, 2020 Jun 15]. J Chin Med Assoc. https://doi.org/10.1097/JCMA.0000000000000375
ClinicalTrials.gov. Estrogen patch for COVID-19 symptoms. Sponsor: Sharon Nachman, Stony Brook University, Identifier: NCT04359329
https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB et al (2020) COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 92(6):577–583
Conti P, Younes A (2020) Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents 34(2):71. https://doi.org/10.23812/Editorial-Conti-3
Zhang P et al (2020) Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 126:1671–1681. https://doi.org/10.1161/CIRCRESAHA.120.317134
Matsuyama R, Nishiura H, Kutsuna S et al (2016) Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health 16(1):1203. https://doi.org/10.1186/s12889-016-3881-4
Wambier CG, Goren A, Vaño-Galván S, et al (2020) Androgen sensitivity gateway to COVID-19 disease severity [published online ahead of print, 2020 may 15]. Drug Dev Res. https://doi.org/10.1002/ddr.21688
Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J (2020) Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care 24(1):108. https://doi.org/10.1186/s13054-020-2833-7
Ludvigsson JF (2020) Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109(6):1088–1095. https://doi.org/10.1111/apa.15270
Brodin P (2020) Why is COVID-19 so mild in children? Acta Paediatr 109(6):1082–1083. https://doi.org/10.1111/apa.15271
Giagulli VA, Guastamacchia E, Magrone T et al (2020) Worse progression of COVID-19 in men: is testosterone a key factor? [published online ahead of print, 2020 Jun 11]. Andrology. https://doi.org/10.1111/andr.12836
Schroeder M et al (2020) The majority of male patients with COVID-19 present low testosterone levels on admission to intensive Care in Hamburg, Germany: a retrospective cohort study. medRxiv:2020
Rastrelli G, Di Stasi V, Inglese F, et al. (2020) Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients [published online ahead of print, 2020 May 20]. Andrology. https://doi.org/10.1111/andr.12821
Wang J, Luo Q, Chen R, Chen T, Li J (2020) Susceptibility analysis of COVID-19 in smokers based on ACE2. Preprints:2020030078. https://doi.org/10.20944/preprints202003.0078.v1
Leung JM, Yang CX, Tam A et al (2020) ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Resp J 55(2000688)
Alraddadi BM, Watson JT, Almarashi A, Abedi GR, Turkistani A, Sadran M, Housa A, Almazroa MA, Alraihan N, Banjar A et al (2016) Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia. Emerg Infect Dis 22:49–55. https://doi.org/10.3201/eid2201.151340
Rossato M, Russo L, Mazzocut S et al (2020) Current smoking is not associated with COVID-19. Eur Respir J 55:2001290. https://doi.org/10.1183/13993003.01290-2020
Richardson S, Hirsch JS, Narasimhan M et al (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323(20):2052–2059. https://doi.org/10.1001/jama.2020.6775
Lippi G, Henry BM (2020) Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med 75:107–108
Halpin DMG, Faner R, Sibila O, Badia JR, Agusti A (2020) Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med 8(5):436–438. https://doi.org/10.1016/S2213-2600(20)30167-3
Goren A, McCoy J, Wambier CG, Vano-Galvan S, Shapiro J, Dhurat R et al (2020) What does androgenetic alopecia have to do with COVID-19? An insight into a potential new therapy. Dermatol Ther:e13365. https://doi.org/10.1111/dth.13365
Ding D, Xu L, Menon M, Reddy GP, Barrack ER (2004) Effect of a short CAG (glutamine) repeat on human androgen receptor function. Prostate. 58(1):23–32. https://doi.org/10.1002/pros.10316
Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M (2018) Testosterone and cardiovascular risk: meta-analysis of interventional studies. J Sex Med 15(6):820–838. https://doi.org/10.1016/j.jsxm.2018.04.641
Schüring AN, Welp A, Gromoll J et al (2012) Role of the CAG repeat polymorphism of the androgen receptor gene in polycystic ovary syndrome (PCOS). Exp Clin Endocrinol Diabetes 120(2):73–179. https://doi.org/10.1055/s-0031-1291343
Moulton VR (2018) Sex hormones in acquired immunity and autoimmune disease. Front Immunol 9:2279. https://doi.org/10.3389/fimmu.2018.02279 Published 2018 Oct 4
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626e638
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271.e8–280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Sharifi N, Ryan CJ (2020) Androgen hazards with COVID-19. Endocr Relat Cancer 27(6):E1–E3. Published 2020 Apr 24. https://doi.org/10.1530/ERC-20-0133
Chakravarty D, Nair SS, Hammouda N et al (2020) Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol 3(1):–374. Published 2020 Jul 8. https://doi.org/10.1038/s42003-020-1088-9
Mjaess G, Karam A, Aoun F, Albisinni S, Roumeguère T (2020) COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor [published online ahead of print, 2020 May 22]. Prog Urol:S1166-7087(20)30186-X. https://doi.org/10.1016/j.purol.2020.05.007
Majdic G (2020) Could sex/gender differences in ACE2 expression in the lungs contribute to the large gender disparity in the morbidity and mortality of patients infected with the SARS-CoV-2 virus? Front Cell Infect Microbiol 10:327. Published 2020 Jun 9. https://doi.org/10.3389/fcimb.2020.00327
Sun K, Gu L, Ma L, Duan Y (2020) Atlas of ACE2 gene expression in mammals reveals novel insights in transmission of SARS-Cov-2. Bio Rxiv. https://doi.org/10.1101/2020.03.30.015644
Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V (2020) COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int J Mol Sci 21(10):3474. https://doi.org/10.3390/ijms21103474
Mikkonen L, Pihlajamaa P, Sahu B, Zhang F, PJänne OA (2010) Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 317(1–2):14–24
Marquez EJ et al (2020) Sexual-dimorphism in human immune system aging. Nat Commun 11:751. https://doi.org/10.1038/s41467-020-14396-9
Carey MA, Card JW, Voltz JW, Arbes SJ Jr, Germolec DR, Korach KS, Zeldin DC (2007) It’s all about sex: gender, lung development and lung disease. Trends in Endocrinol Metab 18(8):308–313
Torday JS, Nielsen HC, Fencl Mde M, Avery ME (1981) Sex differences in fetal lung maturation. Am Rev Respir Dis 123(2):205–208. https://doi.org/10.1164/arrd.1981.123.2.205
Pal R (2020) COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 68(2):251–252. https://doi.org/10.1007/s12020-020-02325-1
Silverman MN, Pearce BD, Biron CA, Miller AH (2005) Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol 18(1):41–78. https://doi.org/10.1089/vim.2005.18.41
Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7(1):a020412. Published 2015 Jan 5. https://doi.org/10.1101/cshperspect.a020412
Massarotti C, Garolla A, Maccarini E (et al, 2020) SARS-CoV-2 in the semen: where does it come from? [published online ahead of print, 2020 Jun 13]. Andrology. https://doi.org/10.1111/andr.12839
Bhasin S, Brito JP, Cunningham GR, et al. (2018) Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 103(5):1715–1744. https://doi.org/10.1210/jc.2018-00229
Dutta S, Sengupta P (2020) SARS-CoV-2 and male infertility: possible multifaceted pathology [published online ahead of print, 2020 Jul 10]. Reprod Sci:1–4. https://doi.org/10.1007/s43032-020-00261-z
Sansone A, Mollaioli D, Ciocca G et al (2020) Addressing male sexual and reproductive health in the wake of COVID-19 outbreak [published online ahead of print, 2020 Jul 13]. J Endocrinol Investig:1–9. https://doi.org/10.1007/s40618-020-01350-1
Chrousos GP, Kaltsas G (2005) Post-SARS sickness syndrome manifestations and endocrinopathy: how, why, and so what? Clin Endocrinol 63:363–365. https://doi.org/10.1111/j.1365-2265.2005.02361.x
Leow MK, Kwek DS, Ng AW, Ong KC, Kaw GJ, Lee LS (2005) Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol 63(2):197–202. https://doi.org/10.1111/j.1365-2265.2005.02325.x
Velayoudom FL, Alwis Wijewickrama PS, Ranathunga HI, Somasundaram N. (2020) Endocrine vigilance in COVID-19. J Pak Med Assoc. 70(Suppl 3)(5):S83-S86. https://doi.org/10.5455/JPMA.16
Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M (2020) Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRxiv. https://doi.org/10.2020/2003.2021.20037267
McLachlan RI (2000) The endocrine control of spermatogenesis. Baillieres Best Pract Res Clin Endocrinol Metab 14(3):345–362. https://doi.org/10.1053/beem.2000.0084
Shen Q, Xiao X, Aierken A, et al (2020) The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection [published online ahead of print, 2020 Jun 28]. J Cell Mol Med. https://doi.org/10.1111/jcmm.15541
Xu J, Qi L, Chi X, et al (2006) Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod 74(2):410–416. https://doi.org/10.1095/biolreprod.105.044776
Wang S, Zhou X, Zhang T et al (2020) The need for urogenital tract monitoring in COVID-19. Nat Rev Urol 17:314–315. https://doi.org/10.1038/s41585-020-0319-7
Zhao S, Zhu W, Xue S, Han D (2014) Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol 11:428–437. https://doi.org/10.1038/cmi.2014.38
Salam AP, Horby PW (2017) The breadth of viruses in human semen. Emerging Infect Dis 23:1922–1924. https://doi.org/10.3201/eid2311.171049
Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O et al (2020) Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. Published online May 29, 2020. https://doi.org/10.1016/j.fertnstert.2020.05.028
Song C, Wang Y, Li W, Hu B, Chen G, Xia P, et al. (2020) Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod 103(1):4–6. https://doi.org/10.1093/biolre/ioaa050
Paoli D, Pallotti F, Colangelo S, Basilico F, Mazzuti L, Turriziani O et al (2020) Study of SARSCoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Investig:1–4. https://doi.org/10.1007/s40618-020-01261-1
Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP (2020) No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril 113:1135–1139. https://doi.org/10.1016/j.fertnstert.2020.04.024
Li D, Jin M, Bao P, Zhao W, Zhang S (2020) Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 3(5):e208292. https://doi.org/10.1001/jamanetworkopen.2020.8292
Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K, Zhou L, Shen S, Liu L, Liu Q, Xiong C (2020) Impaired spermatogenesis in COVID-19 patients. E Clinical Medicine:100604. https://doi.org/10.1016/j.eclinm.2020.100604
Author information
Authors and Affiliations
Contributions
AS and SEB conceived the idea for the article and drafted it; AS and MG performed the literature search, data analysis, and writing process; and AS[D1] and SEB critically revised the work.
Corresponding author
Ethics declarations
Ethical approval
This study was granted exemption from requirement of ethics approval since there is no ethical approval requirement for review articles in the Kocaeli University local ethics committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
All authors give full consent for publication of this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Selek, A., Güçlü, M. & Bolu, Ş.E. COVID-19 pandemic: what about the gonads?. Hormones 20, 259–268 (2021). https://doi.org/10.1007/s42000-021-00277-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-021-00277-3