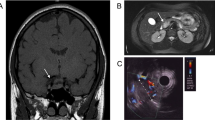
Abstract
Context
Clinical phenotype variability in MEN1 syndrome exists and evidence for an established genotype-phenotype is lacking. However, a higher aggressiveness of MEN1-associated gastro-entero-pancreatic (GEP) (neuro)endocrine tumours (NETs) tumours has been reported when MEN1 gene truncating mutations are detected. We found a novel germline truncating mutation of MEN1 gene at exon 10 in a subject with an aggressive clinical behavior of GEP-NETs. Successively, other two mutant-affected familial members have been identified.
Objective
The aim of this observational study was to investigate genotype-phenotype correlation in these three members, with attention to GPE-NETs behavior over the years.
Design
The genetic and clinical data obtained and the follow-up screening program (2012–2016) were according to the International Guidelines in a multidisciplinary academic reference center. The familial history collected strongly suggested MEN1 GEP-NETs in at least other four members from different generations.
Patients
Three MEN1 patients (aged 30–69 years at MEN1 diagnosis) were clinically screened for MEN1 GEP-NETs, both functioning and nonfunctioning.
Methods
Biochemical, imaging, and nuclear medicine tests and fine-needle agobiopsy were performed, depending on found/emerging clinical symptoms/biochemical abnormalities, and made when necessary.
Results
Our clinical survey found strong genotype-phenotype correlation with aggressive MEN1 GEP-NETs (G1, G2-NETs, and multiple ZES/gastrinomas) over the years. The familial history strongly suggested ZES/gastrinoma in progenitors from previous generations.
Conclusions
This novel MEN1 truncating mutation correlates with an aggressive evolution and behavior of MEN1 GEP-NETs in studied affected subjects, confirming the need for MEN1 individuals to be evaluated by a skilled multidisciplinary team, as also stated by International Guidelines.



Similar content being viewed by others
References
Wermer P. 1963 Endocrine adenomatosis and peptic ulcer in a large kindred. Inherited multiple tumors and mosaic pleiotropism in man. Am J Med;35:205-12
Marx S, Spiegel AM, Skarulis MC et al (1998) Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med 129(6):484–494
Falchetti A 2010 Genetic screening for multiple endocrine neoplasia syndrome type 1 (MEN-1): when and how. F1000 Med Rep;2
Trump D, Farren B, Wooding C et al (1996) Clinical studies of multiple endocrine neoplasia type 1 (MEN1). QJM 89(9):653–669
Flanagan DE, Armitage M, Clein GP et al (1996) Prolactinoma presenting in identical twins with multiple endocrine neoplasia type 1. Clin Endocrinol 45(1):117–120
Namihira H, Sato M, Miyauchi A et al (2000) Different phenotypes of multiple endocrine neoplasia type 1 (MEN1) in monozygotic twins found in a Japanese MEN1 family with MEN1 gene mutation. Endocr J 47(1):37–43
Rix M, Hertel NT, Nielsen FC et al (2004) Cushing’s disease in childhood as the first manifestation of multiple endocrine neoplasia syndrome type 1. Eur J Endocrinol 151(6):709–715
Concolino P, Rossodivita A, Carrozza C et al (2008) A novel MEN1 frameshift germline mutation in two Italian monozygotic twins. Clin Chem Lab Med 46(6):824–826
Brandi ML, Gagel RF, Angeli A et al (2001) Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86(12):5658–5671
Thakker RV, Newey PJ, Walls GV et al (2012) Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 97(9):2990–3011
Nunes VS, Souza GL, Perone D et al (2014) Frequency of multiple endocrine neoplasia type 1 in a group of patients with pituitary adenoma: genetic study and familial screening. Pituitary 17(1):30–37
Triponez F, Sadowski SM, Pattou F, et al. 2017 Long-term follow-up of MEN1 patients who do not have initial surgery for small </=2 cm nonfunctioning pancreatic neuroendocrine tumors, an AFCE and GTE study: Association Francophone de Chirurgie Endocrinienne & Groupe d’Etude des Tumeurs Endocrines. Ann Surg; XX; 1-7
Lourenco DM Jr, Coutinho FL, Toledo RA et al (2010) Early-onset, progressive, frequent, extensive, and severe bone mineral and renal complications in multiple endocrine neoplasia type 1-associated primary hyperparathyroidism. J Bone Miner Res 25(11):2382–2391
Christopoulos C, Antoniou N, Thempeyioti A et al (2005) Familial multiple endocrine neoplasia type I: the urologist is first on the scene. BJU Int 96(6):884–887
de Wilde RF, Edil BH, Hruban RH et al (2012) Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev Gastroenterol Hepatol 9(4):199–208
Dean PG, van Heerden JA, Farley DR et al (2000) Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg 24(11):1437–1441
Goudet P, Bonithon-Kopp C, Murat A et al (2011) Gender-related differences in MEN1 lesion occurrence and diagnosis: a cohort study of 734 cases from the Groupe d'etude des Tumeurs Endocrines. Eur J Endocrinol 165(1):97–105
Marx SJ, Simonds WF (2005) Hereditary hormone excess: genes, molecular pathways, and syndromes. Endocr Rev 26(5):615–661
Teh BT, Zedenius J, Kytola S et al (1998) Thymic carcinoids in multiple endocrine neoplasia type 1. Ann Surg 228(1):99–105
Gibril F, Chen YJ, Schrump DS et al (2003) Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab 88(3):1066–1081
Ferolla P, Falchetti A, Filosso P et al (2005) Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. J Clin Endocrinol Metab 90(5):2603–2609
Goudet P, Murat A, Cardot-Bauters C et al (2009) Thymic neuroendocrine tumors in multiple endocrine neoplasia type 1: a comparative study on 21 cases among a series of 761 MEN1 from the GTE (Groupe des Tumeurs Endocrines). World J Surg 33(6):1197–1207
Schaaf L, Pickel J, Zinner K et al (2007) Developing effective screening strategies in multiple endocrine neoplasia type 1 (MEN 1) on the basis of clinical and sequencing data of German patients with MEN 1. Exp Clin Endocrinol Diabetes 115(8):509–517
Raef H, Zou M, Baitei EY et al (2011) A novel deletion of the MEN1 gene in a large family of multiple endocrine neoplasia type 1 (MEN1) with aggressive phenotype. Clin Endocrinol 75(6):791–800
Hasani-Ranjbar S, Amoli MM, Ebrahim-Habibi A et al (2011) A new frameshift MEN1 gene mutation associated with familial malignant insulinomas. Familial Cancer 10(2):343–348
Concolino P, Costella A, Capoluongo E (2016) Multiple endocrine neoplasia type 1 (MEN1): an update of 208 new germline variants reported in the last nine years. Cancer Genet 209(1–2):36–41
Balogh K, Racz K, Patocs A et al (2006) Menin and its interacting proteins: elucidation of menin function. Trends Endocrinol Metab 17(9):357–364
La P, Desmond A, Hou Z et al (2006) Tumor suppressor menin: the essential role of nuclear localization signal domains in coordinating gene expression. Oncogene 25(25):3537–3546
Kouvaraki MA, Lee JE, Shapiro SE et al (2002) Genotype-phenotype analysis in multiple endocrine neoplasia type 1. Arch Surg 137(6):641–647
Bartsch DK, Langer P, Wild A et al (2000) Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: surgery or surveillance? Surgery 128(6):958–966
Verges B, Boureille F, Goudet P et al (2002) Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab 87(2):457–465
Bartsch DK, Slater EP, Albers M et al (2014) Higher risk of aggressive pancreatic neuroendocrine tumors in MEN1 patients with MEN1 mutations affecting the CHES1 interacting MENIN domain. J Clin Endocrinol Metab 99(11):E2387–E2391
Luzi E, Brandi ML (2011) Are microRNAs involved in the endocrine-specific pattern of tumorigenesis in multiple endocrine neoplasia type 1? Endocr Pract 17(Suppl 3):58–63
Luzi E, Marini F, Tognarini I et al (2010) Ribozyme-mediated compensatory induction of menin-oncosuppressor function in primary fibroblasts from MEN1 patients. Cancer Gene Ther 17(11):814–825
Luzi E, Ciuffi S, Marini F et al (2017) Analysis of differentially expressed microRNAs in MEN1 parathyroid adenomas. Am J Transl Res 9(4):1743–1753
Gurung B, Katona BW, Hua X (2014) Menin-mediated regulation of miRNA biogenesis uncovers the IRS2 pathway as a target for regulating pancreatic beta cells. Oncoscience 1(9):562–566
Lin W, Watanabe H, Peng S, et al. 2015 Dynamic epigenetic regulation by menin during pancreatic islet tumor formation. Mol Cancer Res;13(4):689–698
Acknowledgments
We thank all the patients and their families for participating in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Palermo, A., Capoluongo, E., Del Toro, R. et al. A novel germline mutation at exon 10 of MEN1 gene: a clinical survey and positive genotype-phenotype analysis of a MEN1 Italian family, including monozygotic twins. Hormones 17, 427–435 (2018). https://doi.org/10.1007/s42000-018-0044-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-018-0044-2