Key summary points
To examine the effect of cumulative smoking exposure on the association between peak expiratory flow rate (PEFR) and skeletal muscle mass in middle-aged and older adults.
AbstractSection FindingsThe skeletal muscle mass progressively reduced with decreasing PEFR levels in individuals with non-smoking and light-to-moderate smoking exposure. However, the association between low PEFR level and reduced skeletal muscle mass was not clearly observed in individuals with heavy smoking exposure.
AbstractSection MessageAlthough decreased PEFR in the older population is associated with the age-related loss of systemic skeletal muscle mass, smoking exposure levels of individuals should be taken into consideration when using PEFR as an indicator of sarcopenia.
Abstract
Purpose
This study aimed to examine whether cumulative smoking exposure affects the association between peak expiratory flow rate (PEFR) and skeletal muscle mass in middle-aged and older adults.
Methods
The study participants comprised 832 community-dwelling individuals aged 50–89 years (mean age: 69 years) without chronic obstructive pulmonary disease. Bioelectrical impedance analysis was performed to estimate the skeletal muscle mass of each participant. PEFR was assessed using an electronic spirometer. Cumulative smoking exposure was expressed in pack years, that is a product of the average number of packs of cigarettes smoked per day and smoking duration in years.
Results
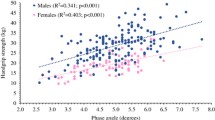
The whole-body skeletal muscle mass progressively reduced with decreasing PEFR levels in both males and females. In the multiple regression analysis, PEFR was found to be significantly associated with skeletal muscle mass, independent of the potential confounding factors. When participants were stratified based on the cumulative smoking exposure, the association between low PEFR and reduced skeletal muscle mass persisted in individuals with non-smoking and light-to-moderate smoking exposure (< 30 pack-years). However, this association was not clearly observed in individuals with heavy smoking exposure (≥ 30 pack-years).
Conclusion
The findings of this study support the notion that PEFR declines with a reduction in systemic skeletal muscle mass due to aging. However, chronic cigarette smoking induces respiratory dysfunction exceeding the expected values by age, and thus a low PEFR level may not be used as a marker of reduced muscle mass in older adults exposed to heavy smoking.

Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Chen LK, Woo J, Assantachai P et al (2020) Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 21:300–307
Elliott JE, Greising SM, Mantilla CB et al (2016) Functional impact of sarcopenia in respiratory muscles. Respir Physiol Neurobiol 226:137–146
Deniz O, Coteli S, Karatoprak NB et al (2021) Diaphragmatic muscle thickness in older people with and without sarcopenia. Aging Clin Exp Res 33:573–580
Kera T, Kawai H, Hirano H et al (2018) Relationships among peak expiratory flow rate, body composition, physical function, and sarcopenia in community-dwelling older adults. Aging Clin Exp Res 30:331–340
Ohara DG, Pegorari MS, Oliveira Dos Santos NL et al (2018) Respiratory muscle strength as a discriminator of sarcopenia in community-dwelling elderly: a cross-sectional study. J Nutr Health Aging 22:952–958
Kera T, Kawai H, Hirano H et al (2019) Definition of respiratory sarcopenia with peak expiratory flow rate. J Am Med Dir Assoc 20:1021–1025
Rom O, Kaisari S, Aizenbud D et al (2012) Identification of possible cigarette smoke constituents responsible for muscle catabolism. J Muscle Res Cell Motil 33:199–208
Degens H, Gayan-Ramirez G, Van Hees HW (2015) Smoking-induced skeletal muscle dysfunction from evidence to mechanisms. Am J Respir Crit Care Med 191:620–625
Hoshino Y, Mio T, Nagai S et al (2001) Cytotoxic effects of cigarette smoke extract on an alveolar type II cell-derived cell line. Am J Physiol Lung Cell Mol Physiol 281:L509–L516
Ohnishi S, Miyai N, Utsumi M et al (2019) Liver fibrosis is associated with loss of skeletal muscle mass in community-dwelling older adults with no history of liver diseases. Jpn J Hyg 74:1–10
Sterling RK, Lissen E, Clumeck N et al (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43:1317–1325
McPherson S, Hardy T, Dufour JF et al (2017) Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 112:740–751
Brinkman GL, Coates EO Jr (1963) The effect of bronchitis, smoking, and occupation on ventilation. Am Rev Respir Dis 87:684–693
Wood DE, Baum SL, Eapen GA et al (2018) Lung cancer screening, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16:412–441
The Ministry of Health, Labor and Welfare (2012) A basic direction for comprehensive implementation of national health promotion, Japan; https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000047330.pdf. Accessed 21 Sep 2021.
Van der Ploeg HP, Chey T, Korda RJ et al (2012) Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med 172:494–500
Tanaka NI, Miyatani M, Masuo Y et al (2007) Applicability of a segmental bioelectrical impedance analysis for predicting the whole body skeletal muscle volume. J Appl Physiol 103:1688–1695
Miyatani M, Kanehisa H, Masuo Y et al (2001) Validity of estimating limb muscle volume by bioelectrical impedance. J Appl Physiol 91:386–394
American thoracic society, European respiratory society (2002) ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med 166:518–624
Schaap LA, Pluijm SMF, Deeg DJH et al (2006) Inflammatory markers and loss of muscle mass (Sarcopenia) and strength. Am J Med 119:526.e9-526.e17
Buchman AS, Boyle PA, Wilson RS et al (2008) Respiratory muscle strength predicts decline in mobility in older persons. Neuroepidemiology 31:174–180
Nogami E, Miyai N, Zhang Y et al (2021) Association of cigarette smoking with muscle mass reduction and low muscle strength in community-dwelling elderly men. Jpn J Hyg 76:1–9
Steffl M, Bohannon RW, Petr M et al (2015) Relation between cigarette smoking and sarcopenia: meta-analysis. Physiol Res 64:419–426
Bian AL, Hu HY, Rong YD et al (2017) A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res 22:25
Rom O, Kaisari S, Aizenbud D et al (2013) The effects of acetaldehyde and acrolein on muscle catabolism in C2 myotubes. Free Radic Biol Med 65:190–200
Bergman BC, Perreault L, Hunerdosse D et al (2012) Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes 61:3156–3166
Kohansal R, Martinez-Canblor P, Agusti A et al (2009) The natural history of chronic airflow obstruction revisited an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 180:3–10
Harmsen L, Thomsen SF, Ingebrigtsen T et al (2010) Chronic mucus hypersecretion: prevalence and risk factors in younger individuals. Int J Tuberc Lung Dis 14:1052–1058
Quanjer PH, Lebowitz MD, Gregg I et al (1997) Peak expiratory flow: conclusions and recommendations of working party of the European Respiratory Society. Eur Respir J 24:2s–8s
Hanai T, Shiraki M, Ohnishi S et al (2016) Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res 46:743–751
Hara N, Iwasa M, Sugimoto R et al (2016) Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med 55:863–870
Onishi S, Miyai N, Zhang Y et al (2021) Excessive alcohol intake and liver fibrosis are associated with skeletal muscle mass reduction in elderly men: the Wakayama study. Aging Clin Exp Res 34:185–192
Sánchez-Sánchez JL, Mañas A, García-García FJ et al (2019) Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: isotemporal substitution model. J Cachexia Sarcopenia Muscle 10:188–198
Smeuninx B, Mckendry J, Wilson D et al (2017) Age-related anabolic resistance of myofibrillar protein synthesis is exacerbated in obese inactive individuals. J Clin Endocrinol Metab 102:3535–3545
Breen L, Stokes KA, Churchward-Venne TA et al (2013) Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 98:2604–2612
Hautmann H, Hefele S, Schotten K et al (2000) Maximal inspiratory mouth pressures (PIMAX) in healthy subjects–what is the lower limit of normal? Respir Med 94:689–693
Funding
This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (JSPS KAKENHI; Grant number: 17K01861).
Author information
Authors and Affiliations
Contributions
EN and NM contributed to the study design, data collection, statistical analysis, interpretation, drafting, and final revision of the manuscript. YZ, SO, and MS contributed to the data collection and drafting of the manuscript. MU and MA contributed to the conception and design of the work and the final revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no potential conflicts of interest with respect to the research, research, authorship, and /or publication of this article.
Ethical approval
All procedures performed in this study were approved by the ethical committee of Wakayama Medical University (approval numbers: G92 and 2975).
Consent to participate
Written informed consent and clearance for the use of examination data were obtained from the participants after explaining the purpose of the study and the experimental procedure.
Consent for publication
The participants approved the manuscript and provided their consent for publication submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nogami, E., Miyai, N., Zhang, Y. et al. Effects of cigarette smoking on the association between respiratory muscle strength and skeletal muscle mass in middle-aged and older adults: the Wakayama Study. Eur Geriatr Med 13, 805–815 (2022). https://doi.org/10.1007/s41999-022-00662-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-022-00662-0