Key summary points
The aim was to examine the predictive value of the record-based MPI in terms of mortality, readmission and LOS.
AbstractSection FindingsThe record-based MPI assessed at discharge predicted mortality and readmission risk in medical inpatients aged ≥ 75 years. Higher MPI risk scores were also associated with longer hospital stay, higher age and female sex.
AbstractSection MessageThe record-based MPI is valuable in predicting mortality and other frailty-related, negative health outcomes in older medical inpatients, and the method is applicable as an alternative to bedside MPI in older hospitalized medical patients.
Abstract
Purpose
To examine the predictive value of the record-based Multidimensional Prognostic Index (MPI) on mortality, readmission and length of hospital stay (LOS) among older medical inpatients.
Methods
A cohort of medical inpatients aged ≥ 75 years was rated using the record-based MPI to assess frailty retrospectively. 90-day and 1-year mortality hazard ratios (HRs) were calculated in a sex- and age-adjusted Cox proportional hazards model. 30-day readmission relative risk (RR) estimates were calculated in a binary regression model with mortality as a competing risk. Discrimination was expressed by the area under the receiver operating characteristic (ROC) curve. Median LOS was calculated using the non-parametric Kruskal–Wallis one-way ANOVA.
Results
In total, 1190 patients with a median age of 83 years were included. 50% were male. 335 patients (28%) were categorized as non-frail (MPI score 0.0–0.33), 522 (44%) moderately frail (MPI score 0.34–0.66) and 333 (28%) severely frail (MPI score 0.67–1.0). 90-day mortality HR was 7.4 (95% confidence interval (CI) 2.9–18.6, p < 0.001) for the moderately frail and 18.5 (95% CI 7.5–46.1, p < 0.001) for the severely frail compared with the non-frail. ROC area was 0.76 (95% CI 0.72–0.80). Similarly, 1-year mortality HR was 3.3 (95% CI 2.2–5.0, p < 0.001) for the moderately frail and 7.1 (95% CI 4.7–10.6, p < 0.001) for the severely frail. 30-day readmission RR was 2.1 (95% CI 1.5–2.9, p < 0.001) for the moderately frail and 1.8 (95% CI 1.3–2.6, p = 0.001) for the severely frail. LOS was significantly longer with increasing MPI score (p < 0.001).
Conclusion
The record-based MPI assessed at discharge predicts dose-dependent post-discharge mortality and readmission risk and is associated with LOS in older medical inpatients.
Similar content being viewed by others
Background
Frailty is a state of increased vulnerability to stressor events [1], commonly encountered among hospitalized older people. Frail older people are at increased risk of negative health events [2, 3], and such events may eventually increase the level of frailty, leading to a vicious circle of further loss of functional capacity, increasing care needs, prolonged hospital admission, readmission, and death [4]. It is crucial to uncover the frailty at hospital discharge to enable initiation of qualified post-discharge interventions directed to counteract and cushion potential frailty impacts. The comprehensive geriatric assessment (CGA) [5, 6] is the established clinical assessment model in geriatric practice. The CGA goes well beyond frailty assessment, including other dimensions as well. The Multidimensional Prognostic Index (MPI) was developed and validated as a CGA-based prognostic tool to assess 1-year mortality risk [7]. It is also valid in predicting other frailty-related negative health outcomes [8,9,10,11,12,13,14,15,16] and has been proposed as a frailty assessment model [17, 18]. The MPI is an eight-item aggregate score based on a bedside assessment of co-habitation status, number of drugs used, activities of daily living, instrumental activities of daily living, cognitive status, pressure sore risk, morbidity and nutritional risk, resulting in a tripartite risk classification. The MPI is subject to further development and refinement [19,20,21]. Recently it has been suggested that a medical record-based MPI may also be feasible and reliable in detecting frailty [22]; however, the record-based MPI's predictive abilities regarding clinical outcomes remain unknown.
The aim was to examine the predictive value of the record-based MPI on mortality, readmission and length of hospital stay (LOS) in a cohort of older medical inpatients.
Materials and methods
Frailty rating
A cohort of acutely admitted medical inpatients was retrospectively frailty rated using the record-based MPI [22]. The record-based MPI builds on the original MPI [7] and is based on co-habitation status, number of drugs used, Functional Recovery Score activities of daily living (FRS-ADL), Functional Recovery Score instrumentalized activities of daily living (FRS-IADL) [23], trichotomised Short Portable Mental Status Questionnaire (SPMSQ) [24], Exton–Smith scale (ESS) [25], Cumulative Illness Rating Scale-geriatrics (CIRS-G) [26] and the Mini Nutritional Assessment-short form (MNA-SF) [27]. The ratings were based on data available in the individual electronic medical records up until the discharge date, including multidisciplinary assessments, and information on preadmission care needs. The record-based MPI rating resulted in an aggregate score ranging 0–1. Based on the previously established cut-off values [7], the score produced a tripartite risk grade classification: score 0–0.33, low risk, MPI grade 1 (MPI = 1); score 0.34–0.66, moderate risk, MPI grade 2 (MPI = 2); and score 0.67–1, severe risk, MPI grade 3 (MPI = 3).
Setting and population
All patients resided in the catchment area of a medium-sized hospital providing secondary care (outpatient-, inpatient- and intensive care) to a total population of 226,731 of whom 9% were aged ≥ 75 years [28]. The hospital’s medical department (MD) has four subspecialized wards, all providing internal medicine services: cardiology, respiratory medicine/rheumatology, gastroenterology/endocrinology, and geriatrics. Internists also deliver round-the-clock service in the emergency department (ED). Inclusion: all medical patients aged ≥ 75 years at admission and hospitalized from January 1, 2019 onwards were consecutively evaluated by a research nurse (SRLB) at discharge from the MD or the ED to primary care. MPI rating was done only in patients who required personal assistance on a daily basis and/or had a Charlson Comorbidity Index (CCI) [29] ≥ 1 according to the registered diagnoses at admission. Exclusion: patients who were receiving palliative care or declared terminally ill at admission and those who were admitted with stroke were excluded due to specialized follow-up programs. Previously excluded patients who were eventually readmitted were re-evaluated and frailty rated if they fulfilled the inclusion- and exclusion criteria. Each individual patient was only included in the cohort once. Inclusion into the cohort continued until the required sample size was achieved. The ratings and outcomes were recorded using a secured web-based data recording system [30, 31]. The main outcome was all-cause post-discharge mortality occurring within 90 days after admission. Secondary outcomes were 30-day all-cause readmissions and total LOS measured in days. Readmission was defined as any unplanned, acute hospitalization occurring later than 4 h and within 30 days after discharge [32]. One-year all-cause mortality was added post hoc as an outcome. Data regarding index admission, readmission and LOS were retrospectively retrieved from the electronic medical records. Mortality data were collected through the Civil Registration System.
Data collection
Sampling and frailty rating were made independently. All eligible patients were included within two days after discharge by the research nurse. The MPI ratings were made consecutively in portions of 5–35 medical records per day by a registrar in geriatric medicine (TKH) trained in the MPI rating process [22].
Statistical analyses
A minimum of 100 events is recommended for external validation of prognostic models [33]. Based on previously reported 90-day mortality rates of 20% among geriatric patients [10, 34], a minimum sample size of 333 patients in each of the three MPI risk grades was considered sufficient to provide robust estimates. Hazard ratios (HRs) for mortality were calculated using the Cox proportional hazards model, and survival was displayed using the Kaplan–Meyer model. Readmissions did not fulfill the Cox proportional hazards assumption, and mortality was a competing risk. Instead, a crude readmission relative risk (RR) and an age- and sex-adjusted RR were calculated in a binary regression model. Cumulative incidence of readmission adjusted for post-discharge mortality was displayed using the Aalen–Johansen estimator. Association between MPI score and age was calculated using chi-squared test, and the association between MPI score and sex using the non-parametric Kruskal–Wallis one-way ANOVA. Comorbidity was part of the exposure; hence no adjustment was made. Discriminative ability was expressed by the area under the receiver operating characteristic (ROC) curve. The ROC area was calculated using the ordinate MPI score as a continuous classification variable in a non-parametric ROC-analysis producing binomial confidence intervals. The median LOS was calculated also using the non-parametric Kruskal–Wallis one-way ANOVA, and displayed with the corresponding MPI risk grades and MPI scores at discharge using box-and-whisker plots. Data analysis was made using Stata, version 16 [35] and a significance level of less than 0.05. Wherever possible, the 95% confidence intervals (CI) of estimates were reported.
Results
In the period from January 1, 2019 to June 21, 2019, 1,190 eligible patients were admitted to and discharged from the MD or the ED because of an acute medical condition. The flow of participants is displayed in Fig. 1.
Eligible patients were older than 75 years, required daily assistance or had a CCI ≥ 1, were admitted for a medical condition and living within the hospital’s uptake area. Non-eligible were patients who received palliative care or were declared terminally ill at admission and those who died during hospitalization. Those who were admitted with stroke or had a CCI < 1 and no need for daily assistance were excluded, but could be included later on in the inclusion period if the eligibility criteria were later fulfilled. The predefined sample size of ≥ 333 patients in each MPI grade was achieved on June 21, 2019.
Of the total cohort, 335 (28%) patients were categorized as non-frail (MPI score 0.0–0.33; MPI risk grade 1), 522 (44%) as moderately frail (MPI score 0.34–0.66; MPI risk grade 2) and 333 (28%) as severely frail (MPI score 0.67–1.0; MPI risk grade 3). The overall median MPI score was 0.4375 (interquartile range 0.3125–0.6875). Age and sex were unevenly distributed, as higher age (p < 0.001) and higher proportions of females (p = 0.002) were seen with higher MPI scores. Further baseline characteristics are listed in Table 1.
Mortality, readmission and LOS
Mortality and readmission rates along with crude and age- and sex-adjusted HRs and RRs are displayed in Table 2.
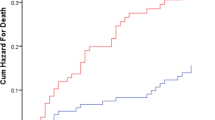
None of the eight MPI items were better at predicting mortality than the aggregated MPI score. The overall 90-day mortality rate was 12.5%; correspondingly, 27.9% died within a year after admission. Male patients had a higher day-to-day mortality hazard than females, reflected in a HR 1.6 (95% CI 1.3–2.0, p < 0.001). Similarly, age increased the mortality HR by approximately 4% per year of age (95% CI 2–6%, p < 0.001). A logistic regression model using the MPI scores (0–1) as exposure produced similar results; however, it did not take the exposure time into consideration. The Cox proportionality of hazards assumption and the Kaplan–Meyer analysis assumptions were all fulfilled. Survival estimates based on the three MPI risk grades are displayed in Fig. 2.
Kaplan–Meyer survival plot showing age- and sex-adjusted 90-day (dotted line) and 1-year mortality with 95% CI in the three MPI risk grades. In-hospital mortality is not included. Abbreviations and legend: MPI Multidimensional Prognostic Index, 95% CI 95% confidence interval, MPI = 1 MPI risk grade 1, MPI = 2 MPI risk grade 2, MPI = 3 MPI risk grade 3
The areas under the ROC curve for 90-day mortality and 1-year mortality as predicted by the MPI score (0–1) were 0.76 (95% CI 0.72–0.80) and 0.73 (95% CI 0.70–0.76), respectively.
Readmission occurred in 21.3% (n = 253) of the cohort. Independently of adjustment, the only single items significantly predictive for 30-day readmission were the FRS-ADL, FRS-IADL and the MNA-SF. The mortality-adjusted cumulative incidence of readmission in the three MPI risk grades is displayed in Fig. 3.
Aalen–Johansen plot showing mortality-adjusted cumulative incidence of acute all-cause readmissions within 30 days after discharge. MPI Multidimensional Prognostic Index, 95% CI 95% confidence interval, MPI = 1 MPI risk grade 1, MPI = 2 MPI risk grade 2, MPI = 3 MPI risk grade 3. Light grey solid lines: cumulative incidence for the MPI risk grade 1. Dark grey solid lines: cumulative incidence for the MPI risk grade 2. Black solid lines: cumulative incidence for the MPI risk grade 3. Short dashed light grey lines: upper and lower 95% CI for the MPI risk grade 1. Dark grey medium long dashed lines: upper and lower 95% CI for the MPI risk grade 2. Black long dashed lines: upper and lower 95% CI for the MPI risk grade 3
The area under the ROC curve for readmission as predicted by the MPI score (0–1) was 0.57 (95% CI 0.53–0.60).
LOS and the MPI were closely related as displayed in “Appendix”. Many patients at moderate and high MPI risk grades were discharged after transitory admissions.
Discussion
In this large cohort study, the record-based MPI measured at discharge showed significant ability in predicting mortality among older medical inpatients. Furthermore, an MPI grade 2 or 3 at discharge was associated with a twofold increased risk of acute readmission. Its discriminative ability regarding mortality was good, whereas its discrimination was poorer with regard to readmission. A strong correlation between the recorded MPI grade and the retrospective LOS was seen, and higher MPI scores were seen in females and with increasing age.
Comparing the results to previous findings based on bedside MPI, we find that the record-based MPI seems equally useful in predicting mortality risk. The observed mortality rates were consistent with the results presented in the original MPI development and validation study [7]; however, in a large prospective multicenter study, Pilotto et al. reported somewhat lower mortality rates among the moderately frail patients [15]. This difference could be attributed to a variation in the age distribution, particularly among the moderately frail; also, patients with stroke, fractures or patients younger than 75 years were not included in our study. Moreover, the record-based MPI enabled us to perform MPI ratings of all eligible patients regardless of their ability to consent. Pilotto et al. [15] reported a good discriminatory ability regarding 1-year mortality risk using bedside MPI obtained at admission (area under the ROC curve = 0.76). When obtained at discharge in a cohort of geriatric patients, the bedside MPI also showed good discriminative ability regarding 90-day mortality, resulting in an area under the ROC curve = 0.74 [10]. Our record-based MPI data showed comparable discrimination. This was surprising considering the possible inaccuracies incorporated in the record-based MPI rating method. Like in previous research, none of the single MPI items outperformed the aggregated score in predicting mortality. Other record-based frailty rating approaches such as the International Statistical Classification of Diseases and Related Health Problems, 10th Revision [36] (ICD-10)-based Hospital Frailty Risk Score (HFRS) [37] and the electronic Frailty Index (eFI) [38, 39] are available; however, the HFRS only assesses coded morbidity, missing out on other frailty elements. Although the eFI, containing 36 deficits, is quite comprehensive and based on a persons’ needs rather than on service use, its use is limited to primary care.
Originally, the MPI was developed to predict mortality, and the predictive ability of the MPI regarding risk of hospital readmission has been reported only to a very limited extent. The association between higher readmission risk and an MPI score ≥ 0.33 observed in our dataset was coherent with the previous results [10, 15]. Fully evaluating the MPI as a prediction model for readmission was beyond the scope of the present study, however, the record-based MPI score does not appear suitable for identifying individuals at risk of readmission. Although the all-cause readmission risk among older medical patients is high, it is important to remember that not all (re)admissions following an index admission are undesired or avoidable. Especially in frail patients, short-term readmission may be a more appropriate outcome measure than long-term readmission [40]. The cumulative incidence of readmission in the low-risk MPI grade was significantly lower than the incidence among patients in the moderate and high-risk grades, suggesting that differentiated post-discharge actions to counteract readmissions according to the MPI risk grade may be beneficial. Further development and validation of multidimensional readmission risk assessment and prediction models for frail people remains an important issue for future research [41,42,43,44]. The strong correlation between higher record-based MPI scores and longer LOS confirmed previous findings based on bedside MPI assessment [14]. Similarly, the known associations between female sex, older age and higher frailty levels were confirmed [7, 45]. Still, frailty cannot be equated with high age.
The record-based MPI rating method applied in our study has some limitations: first, the available medical record material must be considered carefully. The generalizability of the results is limited to in-hospital use as the applied MPI rating method requires comprehensive, multidisciplinary record keeping. The records available in this study provided adequate information to complete the MPI ratings as the ratings were made at discharge; and the medical records always, as a standard, provide recent, intersectoral and multidisciplinary information. Although our data originate from patients admitted to multiple wards for a broad variety of medical diseases, the applicability of the record-based MPI rating method should be generalized with caution. We encourage other researchers to apply, evaluate and further develop the record-based method in other populations, medical record- and health care systems. Second, the MPI rater was not blinded to the outcomes. Due to the large number of patients, it was not possible to rate all records before the outcomes occurred, nor did we have access to a blinded rater dedicated only to collect the outcome data. Nonetheless, we argue that the risk of confirmation bias is minor as the highly structured MPI rating method with its well-defined subdomains is resistant to interrater variation. Furthermore, the actual rater’s reproducibility and accuracy has previously been evaluated [22]. We recommend comparing record-based MPI raters to interdisciplinary bedside-performed MPI rating. Third, the exclusion of self-sufficient individuals with a CCI < 1 may have introduced selection bias. However, the exclusion criteria were very relaxed, only sorting out those who were completely independent and those without severe comorbidity.
The evidence presented in this study unveils the potential for predicting mortality, readmission and LOS using a record-based MPI assessment of frailty among older medical inpatients. Similar to bedside MPI rating, the record-based MPI rating at discharge can support clinical decision making and assessment of an individual's survival prognosis. Our findings imply that a record-based MPI rating of older medical patients’ medical records at discharge may be helpful to timely identify high-risk patient groups, flagging individuals with high mortality risk and groups of patients with high readmission risk. The record-based MPI seems useful whenever multidimensional frailty assessment is needed, and the patient is no longer accessible for bedside assessment.
Availability of data and material
Anonymized data are available on request. To maintain confidentiality, the medical record material is not available. The study protocol (in Danish) is available on request.
Code availability
Stata code is available on request.
References
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381(9868):752–762
Kahlon S, Pederson J, Majumdar SR, Belga S, Lau D, Fradette M et al (2015) Association between frailty and 30-day outcomes after discharge from hospital. Can Med Assoc J 187(11):799–804
Stillman GR, Stillman AN, Beecher MS (2019) Frailty is associated with early hospital readmission in older medical patients. J Appl Gerontol. https://doi.org/10.1177/0733464819894926
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci 56(3):M146–M156
Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH et al (2017) Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006211.pub3
Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K et al (2018) What is comprehensive geriatric assessment (CGA)? An umbrella review. Age Ageing 47(1):149–155
Pilotto A, Ferrucci L, Franceschi M, D’Ambrosio LP, Scarcelli C, Cascavilla L et al (2008) Development and validation of a multidimensional prognostic index for 1-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res 11:151–161
Angleman SB, Santoni G, Pilotto A, Fratiglioni L, Welmer AK (2015) Multidimensional prognostic index in association with future mortality and number of hospital days in a population-based sample of older adults: results of the EU Funded MPI-AGE project. PLoS ONE 10(7):89. https://doi.org/10.1371/journal.pone.0133789
Cruz-Jentoft AJ, Daragjati J, Fratiglioni L, Maggi S, Mangoni AA, Mattace-Raso F et al (2020) Using the Multidimensional Prognostic Index (MPI) to improve cost-effectiveness of interventions in multimorbid frail older persons: results and final recommendations from the MPI_AGE European Project. Aging Clin Exp Res 32(5):861–868
Gregersen M, Hansen TK, Jørgensen BB, Damsgaard EM (2020) Frailty is associated with hospital readmission in geriatric patients: a prognostic study. Eur Geriatr Med. https://doi.org/10.1007/s41999-020-00335-w
Meyer AM, Becker I, Siri G, Brinkkötter PT, Benzing T, Pilotto A et al (2019) New associations of the Multidimensional Prognostic Index. Zeitschrift Gerontol Geriatr 52(5):460–467
Pata G, Bianchetti L, Rota M, Marengoni A, Chiesa D, Cassinotti E et al (2020) Multidimensional Prognostic Index (MPI) score has the major impact on outcome prediction in elderly surgical patients with colorectal cancer: the FRAGIS study. J Surg Oncol. https://doi.org/10.1002/jso.26314
Pilotto A, Rengo F, Marchionni N, Sancarlo D, Fontana A, Panza F et al (2012) Comparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patients. PLoS ONE 7:1–9
Pilotto A, Sancarlo D, Pellegrini F, Rengo F, Marchionni N, Volpato S et al (2016) The Multidimensional Prognostic Index predicts in-hospital length of stay in older patients: a multicentre prospective study. Age Ageing 45(1):90–96
Pilotto A, Veronese N, Daragjati J, Cruz-Jentoft A, Polidori MC, Mattace-Raso F et al (2019) Using the Multidimensional Prognostic Index to predict clinical outcomes of hospitalized older persons: a prospective, multicenter, international study. J Gerontol Ser A Biol Sci Med Sci 74:1643–1649
Volpato S, Bazzano S, Fontana A, Ferrucci L, Pilotto A (2015) Multidimensional Prognostic Index predicts mortality and length of stay during hospitalization in the older patients: a multicenter prospective study. J Gerontol Ser A Biol Sci Med Sci 70:325–331
Dent E, Kowal P, Hoogendijk EO (2016) Frailty measurement in research and clinical practice: a review. Eur J Intern Med 31:3–10
Warnier RMJ, van Rossum E, van Velthuijsen E, Mulder WJ, Schols JMGA, Kempen GIJM (2016) Validity, reliability and feasibility of tools to identify frail older patients in inpatient hospital care: a systematic review. J Nutr Health Aging 20:218–230
Cella A, Ferrari A, Rengo G, Solfrizzi V, Veronese N, Puntoni M et al (2020) Agreement of a Short Form of the Self-Administered Multidimensional Prognostic Index (SELFY-MPI-SF): a useful tool for the self-assessment of frailty in community-dwelling older people. Clin Interv Aging 15:493–499
Pilotto A, Custodero C, Maggi S, Polidori MC, Veronese N, Ferrucci L (2020) A multidimensional approach to frailty in older people. Ageing Res Rev 60:98. https://doi.org/10.1016/j.arr.2020.101047
Pilotto A, Veronese N, Guerrero KLQ, Zora S, Boone ALD, Puntoni M et al (2019) Development and validation of a self-administered Multidimensional Prognostic Index to predict negative health outcomes in community-dwelling persons. Rejuvenation Res 22:299–305
Hansen TK, Damsgaard EM, Shahla S, Bruun JM, Gregersen M (2020) A reliable and record-based frailty assessment method for older medical inpatients. Eur Geriatr Med. https://doi.org/10.1007/s41999-020-00345-8
Zuckerman JD, Koval KJ, Aharonoff GB, Skovron ML (2000) A functional recovery score for elderly hip fracture patients: II. Validity and reliability. J Orthop Trauma 14:26–30
Pfeiffer E (1975) A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 23(10):433–441
Bliss MR, McLaren R, Exton-Smith AN (1966) Mattresses for preventing pressure sores in geriatric patients. Mon Bull Ministry Health Public Health Lab Serv 25:238–268
Miller MD, Towers A (1991) A manual of guidelines for scoring the Cumulative Illness Rating Scale for Geriatrics (CIRS-G). p 31
Sancarlo D, D’Onofrio G, Franceschi M, Scarcelli C, Niro V, Addante F et al (2011) Validation of a modified-multidimensional prognostic index (m-MPI) including the mini nutritional assessment short-form (MNA-SF) for the prediction of 1-year mortality in hospitalized elderly patients. J Nutr Health Aging 15:169–173
Statistikbanken (2019) Statistics Denmark. https://www.statistikbanken.dk/FOLK1A. Accessed 19 June 2020
Charlson ME, Pompei P, Ales KA, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L et al (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208. https://doi.org/10.1016/j.jbi.2019.103208
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Sundhedsstyrelsen MMT (2009) Genindlæggelser af ældre i Danmark 2008. København 2009
Collins GS, Ogundimu EO, Altman DG (2016) Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med 35(2):214–226
Pedersen LH, Gregersen M, Barat I, Damsgaard EM (2017) Early geriatric follow-up after discharge reduces mortality among patients living in their own home. A randomised controlled trial. Eur Geriatr Med 8(4):330–336
StataCorp (2019) Stata Statistical Software. Release 16. College Station, StataCorp LCC
WHO (2010) International Statistical Classification of Diseases and Related Health Problems 10th Revision. World Health Organization. https://icd.who.int/browse10/2010/en. Accessed 17 Aug 2020
Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C et al (2018) Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 391:1775–1782
Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E et al (2016) Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 45:353–360
Clegg A, Bates C, Young J, Ryan R, Nichols L, Teale E et al (2017) Development, internal validation and independent external validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 46:i35. https://doi.org/10.1093/ageing/afx068.129
Heggestad T, Lilleeng SE (2003) Measuring readmissions: focus on the time factor. Int J Qual Health Care 15(2):147–154
Andreasen J, Aadahl M, Sørensen EE, Eriksen HH, Lund H, Overvad K (2018) Associations and predictions of readmission or death in acutely admitted older medical patients using self-reported frailty and functional measures. A Danish cohort study. Arch Gerontol Geriatr 76:65–72
Artetxe A, Beristain A, Graña M (2018) Predictive models for hospital readmission risk: a systematic review of methods. Comput Methods Progr Biomed 164:49–64
Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M et al (2011) Risk prediction models for hospital readmission: a systematic review. J Am Med Assoc 306(15):1688–1698
Wou F, Gladman JR, Bradshaw L, Franklin M, Edmans J, Conroy SP (2013) The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing 42(6):776–781
Veronese N, Siri G, Cella A, Daragjati J, Cruz-Jentoft AJ, Polidori MC et al (2019) Older women are frailer, but less often die than men: a prospective study of older hospitalized people. Maturitas 128:81–86
Acknowledgements
We would like to thank the multidisciplinary staff in the MD, ED and the four municipalities for their meticulous medical record-keeping and intersectoral cooperation.
Funding
This work was part of a PhD project supported by A.P. Møller Fonden (DKK 45,000), Helsefonden (DKK 350,000) and the Health Research Fund of Central Denmark Region (DKK 100,000).
Author information
Authors and Affiliations
Contributions
The authors fulfil the ICMJE criteria for authorship. Study concept and design: Troels Kjærskov. Hansen (TKH), Seham Shahla (SS), Else Marie Damsgaard (EMD), Sofie Ran Lindhardt Bossen (SRLB), Jens Meldgaard Bruun (JMB), Merete Gregersen (MG); Acquisition of data: TKH, SRLB; Analysis and interpretation of data: TKH, SS, EMD, SRLB, JMB, MG.
Corresponding author
Ethics declarations
Conflict of interest
The authors had no conflicts of interest.
Ethics approval
The project was approved as a quality development project by the Regional Research Ethics Committee (197/2017) and the local hospital administration; hence, no further approval or consent was needed.
Informed consent
No patient received less than usual care, any extra treatment, examination or exposure because of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Fig. 4.
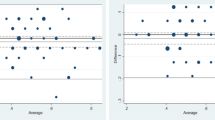
Box-and-whiskers plots showing median LOS as function of MPI risk grade 1, 2 and 3 (left) and the aggregated MPI score (right). The overall median LOS was 3 days. LOS was not significantly affected by sex (p = 0.73). The Kruskal–Wallis hypothesis test showed significantly longer LOS in older patients (p = 0.037), with increasing MPI risk grades (p < 0.001), also when the analysis was based on the MPI score (p < 0.001). MPI Multidimensional Prognostic Index, LOS length of hospital stays. The plots show the mean values (line in box), interquartile ranges (box), and upper and lower values within 1.5 times the interquartile ranges (whiskers), as well as outliers beyond the whiskers (circles). Four outliers had a LOS > 30 days (located outside the plotted area); their LOS were 95 days (MPI = 2); 41 days (MPI = 2); 37 days (MPI = 3) and 34 days (MPI = 3). Only three patients had a MPI score = 1
Rights and permissions
About this article
Cite this article
Hansen, T.K., Shahla, S., Damsgaard, E.M. et al. Mortality and readmission risk can be predicted by the record-based Multidimensional Prognostic Index: a cohort study of medical inpatients older than 75 years. Eur Geriatr Med 12, 253–261 (2021). https://doi.org/10.1007/s41999-021-00453-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-021-00453-z