Abstract
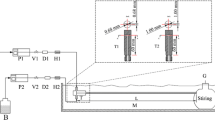
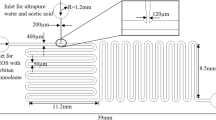
This study presents a robust and scalable method for synthesizing continuously the silica microspheres via the Stöber method. Owing to the superb mixing accessible with gas-liquid segmented flow in coiled microtube, satisfactory morphology and mono-dispersity of silica spheres was obtained. The continuous method was firstly optimized through investigating the effects of various operation parameters on the morphology, particle size, size distribution and chemical structure of silica spheres. Without the surfactant, the size of silica spheres was limited < 850 nm due to the partial blockage of microchannel when synthesizing larger silica spheres. The addition of surfactant migrated the clogging issue, enabling the reliable synthesis of microspheres with diameters over 1 μm within a four times shorter residence time (30 min). Moreover, the mono-dispersity of silica microspheres could be further improved by increasing the gas/liquid ratio in segmented flow due to the more intensified mixing in liquid segments, as verified in visual flow field investigation.















Similar content being viewed by others
References
Hyde EDER, Seyfaee A, Neville F, Moreno-Atanasio R (2016) Colloidal silica particle synthesis and future industrial manufacturing pathways: a review. Ind Eng Chem Res 55(33):8891–8913. https://doi.org/10.1021/acs.iecr.6b01839
Kim JM, Chang SM, Kong SM, Jinsoo K, Kim I-H, Kim K-S, Kim W-S (2008) Method for mono-dispersed large spherical particles of silica for LCD spacer. Mol Cryst Liq Cryst 492:245–256. https://doi.org/10.1080/15421400802330796
Stober W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in micron size range. J Colloid Interface Sci 26(1):62. https://doi.org/10.1016/0021-9797(68)90272-5
Bogush GH, Tracy MA, Zukoski CF (1988) Preparation of monodisperse silica particles - control of size and mass fraction. J Non Cryst Solids 104(1):95–106. https://doi.org/10.1016/0022-3093(88)90187-1
Büchel G, Unger KK, Matsumoto A, Tsutsumi K (1998) A novel pathway for synthesis of Submicrometer-size solid core/mesoporous shell silica spheres. Adv Mater 10(13):1036–1038. https://doi.org/10.1002/(SICI)1521-4095(199809)10:13<1036::AID-ADMA1036>3.0.CO;2-Z
Wang X-D, Shen Z-X, Sang T, Cheng X-B, Li M-F, Chen L-Y, Wang Z-S (2010) Preparation of spherical silica particles by Stober process with high concentration of tetra-ethyl-orthosilicate. J Colloid Interface Sci 341(1):23–29. https://doi.org/10.1016/j.jcis.2009.09.018
Yokoi T, Sakamoto Y, Terasaki O, Kubota Y, Okubo T, Tatsumi T (2006) Periodic arrangement of silica nanospheres assisted by amino acids. J Am Chem Soc 128(42):13664–13665. https://doi.org/10.1021/ja065071y
Bari AH, Jundale RB, Kulkarni AA (2020) Understanding the role of solvent properties on reaction kinetics for synthesis of silica nanoparticles. Chem Eng J 398:10. https://doi.org/10.1016/j.cej.2020.125427
Hristov DR, Mahon E, Dawson KA (2015) Controlling aqueous silica nanoparticle synthesis in the 10–100 nm range. Chem Commun 51(98):17420–17423. https://doi.org/10.1039/c5cc06598d
Luo G, Du L, Wang Y, Wang K (2019) Recent developments in microfluidic device-based preparation, functionalization, and manipulation of nano- and micro-materials. Particuology 45:1–19. https://doi.org/10.1016/j.partic.2018.10.001
Sui J, Yan J, Liu D, Wang K, Luo G (2020) Continuous synthesis of nanocrystals via flow chemistry technology. Small 16(15):1902828. https://doi.org/10.1002/smll.201902828
Ogihara T, Ikeda M, Kato M, Mizutani N (1989) Continuous processing of monodispersed titania powders. J Am Ceram Soc 72(9):1598–1601. https://doi.org/10.1111/j.1151-2916.1989.tb06288.x
Uson L, Arruebo M, Sebastian V, Santamaria J (2018) Single phase microreactor for the continuous, high-temperature synthesis of < 4 nm superparamagnetic iron oxide nanoparticles. Chem Eng J 340:66–72. https://doi.org/10.1016/j.cej.2017.12.024
Kubo M, Yonemoto T (1999) Continuous synthesis of TiO2 fine particles and increase of particle size using a two-stage slug flow tubular reactor. Chem Eng Res Des 77(A4):335–341. https://doi.org/10.1205/026387699526278
Cabeza VS, Kuhn S, Kulkarni AA, Jensen KF (2012) Size-controlled flow synthesis of gold nanoparticles using a segmented flow microfluidic platform. Langmuir 28(17):7007–7013. https://doi.org/10.1021/la205131e
Khan SA, Gunther A, Schmidt MA, Jensen KF (2004) Microfluidic synthesis of colloidal silica. Langmuir 20(20):8604–8611. https://doi.org/10.1021/la0499012
He P, Greenway G, Haswell SJ (2011) Microfluidic synthesis of silica nanoparticles using polyethylenimine polymers. Chem Eng J 167(2–3):694–699. https://doi.org/10.1016/j.cej.2010.08.079
Gutierrez L, Gomez L, Irusta S, Arruebo M, Santamaria J (2011) Comparative study of the synthesis of silica nanoparticles in micromixer-microreactor and batch reactor systems. Chem Eng J 171(2):674–683. https://doi.org/10.1016/j.cej.2011.05.019
Su M (2017) Synthesis of highly monodisperse silica nanoparticles in the microreactor system. Korean J Chem Eng 34(2):484–494. https://doi.org/10.1007/s11814-016-0297-x
Ren GY, Su HJ, Wang SD. The combined method to synthesis silica nanoparticle by Stober process. J Sol-Gel Sci Technol:13. https://doi.org/10.1007/s10971-020-05322-y
Hartman RL, Naber JR, Zaborenko N, Buchwald SL, Jensen KF (2010) Overcoming the challenges of solid bridging and constriction during Pd-catalyzed C-N bond formation in microreactors. Org Process Res Dev 14(6):1347–1357. https://doi.org/10.1021/op100154d
Hartman RL (2012) Managing solids in microreactors for the upstream continuous processing of fine chemicals. Org Process Res Dev 16(5):870–887. https://doi.org/10.1021/op200348t
Barajas AM, Panton RL (1993) The effects of contact-angle on 2-phase flow in capillary tubes. Int J Multiphase Flow 19(2):337–346. https://doi.org/10.1016/0301-9322(93)90007-h
Lee CY, Lee SY (2008) Pressure drop of two-phase plug flow in round mini-channels: Influence of surface wettability. Exp Therm Fluid Sci 32(8):1716–1722. https://doi.org/10.1016/j.expthermflusci.2008.06.007
Lewis JM, Wang Y (2019) Two-phase frictional pressure drop in a thin mixed-wettability microchannel. Int J Heat Mass Transf 128:649–667. https://doi.org/10.1016/j.ijheatmasstransfer.2018.09.010
Lee CY, Lee SY (2010) Pressure drop of two-phase dry-plug flow in round mini-channels: Effect of moving contact line. Exp Therm Fluid Sci 34(1):1–9. https://doi.org/10.1016/j.expthermflusci.2009.08.005
Sen N, Koli V, Singh KK, Panicker L, Sirsam R, Mukhopadhyay S, Shenoy KT (2018) Segmented microfluidics for synthesis of BaSO4 nanoparticles. Chem Eng Process 125:197–206. https://doi.org/10.1016/j.cep.2018.01.012
Dong Z, Fernandez Rivas D, Kuhn S (2019) Acoustophoretic focusing effects on particle synthesis and clogging in microreactors. Lab Chip 19(2):316–327. https://doi.org/10.1039/c8lc00675j
Dong ZY, Udepurkar AP, Kuhn S (2020) Synergistic effects of the alternating application of low and high frequency ultrasound for particle synthesis in microreactors. Ultrason Sonochem 60:9. https://doi.org/10.1016/j.ultsonch.2019.104800
Knossalla J, Mezzavilla S, Schuth F (2016) Continuous synthesis of nanostructured silica based materials in a gas-liquid segmented flow tubular reactor. New J Chem 40(5):4361–4366. https://doi.org/10.1039/c5nj03033a
Han YD (2018) Investigations on growth mechanism and controlled syntheses of silica particles in Stöber method. Jilin University, Changchun
El Rassy H, Pierre AC (2005) NMR and IR spectroscopy of silica aerogels with different hydrophobic characteristics. J Non-Cryst Solids 351(19–20):1603–1610. https://doi.org/10.1016/j.jnoncrysol.2005.03.048
Dubey RS, Rajesh YBRD, More MA (2015) Synthesis and characterization of SiO2 nanoparticles via sol-gel method for industrial applications. Mater Today Proc 2(4–5):3575–3579. https://doi.org/10.1016/j.matpr.2015.07.098
Guo Q, Yang G, Huang D, Cao W, Ge L, Li L (2018) Synthesis and characterization of spherical silica nanoparticles by modified Stober process assisted by slow-hydrolysis catalyst. Colloid Polym Sci 296(2):379–384. https://doi.org/10.1007/s00396-017-4260-0
Moon S, Lee KJ (2017) Simultaneous control of size and surface functionality of silica particle via growing method. Adv Powder Technol 28(11):2914–2920. https://doi.org/10.1016/j.apt.2017.08.019
Ramachandran V, Fogler HS (1999) Plugging by hydrodynamic bridging during flow of stable colloidal particles within cylindrical pores. J Fluid Mech 385:129–156. https://doi.org/10.1017/s0022112098004121
Huang Y, Pemberton JE (2010) Synthesis of uniform, spherical sub-100 nm silica particles using a conceptual modification of the classic LaMer model. Colloid Surf A-Physicochem Eng Asp 360(1–3):175–183. https://doi.org/10.1016/j.colsurfa.2010.02.031
Tan CG, Bowen BD, Epstein N (1987) Production of monodisperse colloidal silica spheres: Effect of temperature. J Colloid Interface Sci 118(1):290–293. https://doi.org/10.1016/0021-9797(87)90458-9
Meier M, Ungerer J, Klinge M, Nirschl H (2018) Synthesis of nanometric silica particles via a modified Stöber synthesis route. Colloids Surf A 538:559–564. https://doi.org/10.1016/j.colsurfa.2017.11.047
Schmutzler T, Schindler T, Schmiele M, Appavou MS, Lages S, Kriele A, Gilles R, Unruh T (2018) The influence of n-hexanol on the morphology and composition of CTAB micelles. Colloid Surf A-Physicochem Eng Asp 543:56–63. https://doi.org/10.1016/j.colsurfa.2017.12.039
Gunther A, Khan SA, Thalmann M, Trachsel F, Jensen KF (2004) Transport and reaction in microscale segmented gas-liquid flow. Lab Chip 4(4):278–286. https://doi.org/10.1039/b403982c
Song H, Tice JD, Ismagilov RF (2003) A microfluidic system for controlling reaction networks in time. Angew Chem Int Ed 42(7):768–772. https://doi.org/10.1002/anie.200390203
Acknowledgements
This work was supported by the Youth Innovation Promotion Association of Chinese Academy of Sciences and the Key Technical Personnel of Chinese Academy of Sciences, the Ministry of Science and Technology of China (grant 2016YFA0602603). The work was also supported by Frontier Scientific Research Project funded by Shell under contract No. PT19253.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Synthesis of monodisperse silica microspheres over 1 μm by gas-liquid segmented flow in microreactor.
• Adding the surfactant solved the clogging problem, and preserved the quality of synthesized silica microspheres.
• Adjusting the gas/liquid flow ratio affected the particle size distribution.
Rights and permissions
About this article
Cite this article
Fei, S., Zhang, Y., Zhang, J. et al. Continuous synthesis of monodisperse silica microspheres over 1 μm size. J Flow Chem 11, 831–842 (2021). https://doi.org/10.1007/s41981-021-00157-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-021-00157-2