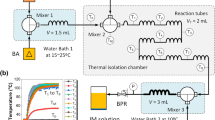
Abstract
High exothermicity, unstable intermediates, high reaction rates are the features that make metallorganic reactions very challenging, especially in commercial operation. No wonder that there is a large interest for the alternative production technology. This paper reviews a research program by Bayer on metallorganic reactions in microreactors. Selected aspects of use of micro-reaction technology for this reaction class are discussed. Two operational issues, i.e. temperature control and clogging are highlighted. Furthermore, the design concept of a MRT based commercial unit and its economics are discussed.











Similar content being viewed by others
References
Wolf A, Michele V, Schlüter OF-K, Herbstritt F, Heck J, Mleczko L (2015). Chem Eng Technol 38(11):2017–2024
Fu M, Luan W, Tu S-T, Mleczko L (2015) J Nanomater 842365/1–842365/9
Lu H, Hoheisel W, Mleczko L, Nowak S Continuous synthesis of high quantum yield InP/ZnS nanocrystals. (2014) EP2785897A1, Oct.
Henig M (2011) Process, 2011. http://www.process-worldwide.com. Accessed 10/20/2010
Buchholz S, Mleczko L (2008). VDI-Ber 2039:177–181
Ji G, Ding C, Zhang G, Ji S, Ji G, Ji X, Yang H (2014) Method utilizing micro-channel to prepare tris(2-chloroethyl)phosphite. CN104119374A, Oct
Mleczko L, Zhao D (2015) Technology for Continuous Production of Fine Chemicals, A Case Study for Low Temperature Lithiation Reactions. In: Managing Hazardous Reactions and Compounds in Process Chemistry; Pesti, J. A., Abdel-Magid, A. F. (Eds). American Chemical Society: Washington, DC
Tian S, Fu M, Hoheisel W, Mleczko L (2016). Chem Eng J 289:365–373
Thaisrivongs DA, Naber JR, McMullen JP (2016). Org Process Res Dev 20:1997–2004
Murray PRD, Browne DL, Pastre JC, Butters C, Guthrie D, Ley SV (2013). Org Process Res Dev 17:1192–1108
Yoshida J (2009) Flash Chemistry: Fast Organic Synthesis in Microsystems. Wiley:New York,
Kim H, Min K-I, Inoue K, Im DJ, Kim D-P, Yoshida J-i (2016). Science 352:691–694
Yoshida J-i, Takahashi Y, Nagaki A (2013). Chem Commun 49:9896–9904
Nagaki A, Ichinari D, Yoshida J-i (2014). J Am Chem Soc 136(35):12245–12448
Hafner A, Meisenbach M, Sedelmeier J (2016). Org Lett 18(15):3630–3633
Hafner A, Filipponi P, Piccioni L, Meisenbach M, Schenkel B, Venturoni F, Sedelmeier J (2016). Org Process Res Dev 20:1833–1837
Laue S, Haverkamp V, Mleczko L (2016). Org Process Res Dev 20:480–486
Xie D, Zhou J, Tian S, Mleczko L, Zhou X (2016). Chem Eng Technol 39(8):1451–1456
Laue S, Haverkamp V, Frye M, Michele V, Mleczko L (2007) Process for continuously preparing difluorobenzene derivatives with long operating times. WO2007054213A1, May
Liu T, Yu F (2010) Continuous reacting device and method for strong exothermic reaction. CN101757881A, June
Westermann T, Mleczko L (2016). Org Process Res Dev 20(2):487–494
Harrington PJ (2011) Pharmaceutical process chemistry for synthesis: rethinking the routes to scale-up. Wiley: New York, pp 304–305
Oppenheimer J Methods of isolating 4-chloro-2-fluoro-3-substituted-phenylboronic acids. (2014) US8822730B2, Sep
Hessel V, Kralisch D, Kockmann N (2015) Novel process windows. Wiley-VCH, Weinheim
Nogaki A, Yoshida J-I (2014) In: Luisi R, Capriati V (eds) Lithium compounds in organic synthesis: from fundamentals to applications. Wiley-VCH, Weinheim
Hunter SM, Susanne F, Whitten R, Hartwig T, Schilling M (2018). Tetrahedron 74:3176–3182
Haber J, Jiang B, Maeder T, Borhani N, Thome J, Renken A, Kiwi-Minskera L (2014). Chem Eng Process Process Intensif 84:14–23
Hartman RL (2012). Org Process Res Dev 16(5):870–887
Flowers BS, Hartman RL (2012). Challenges 3(2):194–211
Hartman RL, Naber JR, Zaborenko N (2010). Org Process Res Dev 14(6):1347–1357
Stubblefield CB, Bach RO (1972). J Chem Eng Data 17(4):491–492
Wynn DA, Roth MM, Pollard BD (1984). Talanta 31(11):1036–1040
Jennifer J (2010) PhD thesis, Universite Francois-Rabelais de Tours
Jensen KF (2017). AICHE J 63(3):858–869
Poechlauer P, Colberg J, Fisher E, Jansen M, Johnson MD, Koenig SG, Lawler M, Laporte T, Manley J, Martin B, O’Kearney-McMullan A (2013). Org Process Res Dev 17:1472–1478
Roberge DM, Ducry L, Bieler N, Cretton P, Zimmermann B (2005). Chem Eng Technol 28(3):318–323
Roberge DM, Zimmermann B, Rainone F, Gottsponer M, Eyholzer M, Kockmann N (2008). Org Process Res Dev 12:905–910
Krtschila U, Hessel V, Kralischd D, Kreiseld G, Küpperb M, Schenkb R (2006). Chimia 60:611–617
Acknowledgments
In this paper we summarized results obtained over the long time by a large team of colleagues. We can’t name them all but we would like to thank explicitly Dres S. Peper who analyzed the solubility of Li-salts, T. Westermann who studied temperature control and Shizhe Tian who dealt with economic evaluation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, M., Mleczko, L. Metallorganic reactions in the polytropic microreactors. J Flow Chem 9, 89–100 (2019). https://doi.org/10.1007/s41981-019-00030-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00030-3