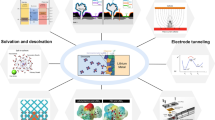
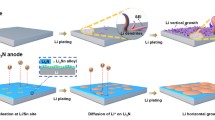
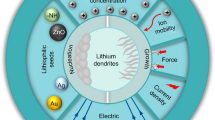
Abstract
Lithium metal anodes (LMAs) show unique superiority for secondary batteries because they possess the lowest molar mass and reduction potential among metallic elements. It can diminish the large gap in energy density between secondary batteries and fossil fuels. However, notorious dendrite propagation gives rise to large volume expansion, low reversibility and potential safety hazards, making the commercial application of LMAs a perennial challenge. The booming development in material characterization deepens the understanding of the dendrite formation mechanism, and the great progress made via nanotechnology-based solutions hastens practical procedures. In this paper, we highlight the current understanding of lithium dendrites. We first illustrate different nucleation theories and growth patterns of lithium dendrites. According to the growth patterns, we classify dendrites into three categories to accurately describe their different formation mechanisms. Then, we concentrate on the factors that may lead to dendritic deposits in each electroplating step. The dendritic morphology originates from the inhomogeneity of Li atoms, electrons, mass transport in the bulk electrolyte and the solid electrolyte interphase. Different inducements lead to different growth patterns. Based on this understanding, strategies for controlling lithium plating are divided into five methodologies. Reasonable integration of the strategies is expected to provide new ideas for basic research and practical application of LMAs. Finally, current limitations and advice for future research are proposed, aiming at inspiring engaged contributors and new entrants to explore scalable solutions for early realization of industrialization.






























Similar content being viewed by others
References
Armand, M., Tarascon, J.M.: Building better batteries. Nature 451, 652–657 (2008). https://doi.org/10.1038/451652a
Tarascon, J.M., Armand, M.: Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). https://doi.org/10.1038/35104644
Dunn, B., Kamath, H., Tarascon, J.M.: Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011). https://doi.org/10.1126/science.1212741
Davis, S.J., Caldeira, K., Matthews, H.D.: Future CO2 emissions and climate change from existing energy infrastructure. Science 329, 1330–1333 (2010). https://doi.org/10.1126/science.1188566
Goodenough, J.B., Park, K.S.: The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013). https://doi.org/10.1021/ja3091438
Stambouli, A.B., Traversa, E.: Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 6, 433–455 (2002). https://doi.org/10.1016/S1364-0321(02)00014-X
Song, Y.Z., Liu, X., Ren, D.S., et al.: Simultaneously blocking chemical crosstalk and internal short circuit via gel-stretching derived nanoporous non-shrinkage separator for safe lithium-ion batteries. Adv. Mater. 34, e2106335 (2022). https://doi.org/10.1002/adma.202106335
Whittingham, M.S.: Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 114, 11414–11443 (2014). https://doi.org/10.1021/cr5003003
Leng, J., Wang, Z.X., Wang, J.X., et al.: Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 48, 3015–3072 (2019). https://doi.org/10.1039/c8cs00904j
Fang, C.C., Wang, X.F., Meng, Y.S.: Key issues hindering a practical lithium-metal anode. Trends Chem. 1, 152–158 (2019). https://doi.org/10.1016/j.trechm.2019.02.015
Guan, X.Z., Wang, A.X., Liu, S., et al.: Controlling nucleation in lithium metal anodes. Small 14, 1801423 (2018). https://doi.org/10.1002/smll.201801423
Kim, H., Jeong, G., Kim, Y.U., et al.: Metallic anodes for next generation secondary batteries. Chem. Soc. Rev. 42, 9011–9034 (2013). https://doi.org/10.1039/C3CS60177C
Zhang, X., Yang, Y., Zhou, Z.: Towards practical lithium-metal anodes. Chem. Soc. Rev. 49, 3040–3071 (2020). https://doi.org/10.1039/c9cs00838a
Liu, J., Bao, Z., Cui, Y., et al.: Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019). https://doi.org/10.1038/s41560-019-0338-x
Bruce, P.G., Freunberger, S.A., Hardwick, L.J., et al.: Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012). https://doi.org/10.1038/nmat3191
Cheng, X.B., Zhang, R., Zhao, C.Z., et al.: Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017). https://doi.org/10.1021/acs.chemrev.7b00115
Wang, A., Kadam, S., Li, H., et al.: Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. NPJ Comput. Mater. 4, 15 (2018). https://doi.org/10.1038/s41524-018-0064-0
Xu, K.: Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004). https://doi.org/10.1021/cr030203g
Wang, H.S., Liu, Y.Y., Li, Y.Z., et al.: Lithium metal anode materials design: interphase and host. Electrochem. Energy Rev. 2, 509–517 (2019). https://doi.org/10.1007/s41918-019-00054-2
Tewari, D., Mukherjee, P.P.: Mechanistic understanding of electrochemical plating and stripping of metal electrodes. J. Mater. Chem. A7, 4668–4688 (2019). https://doi.org/10.1039/c8ta11326b10.1039/c8ta11326b
Lin, D., Liu, Y., Cui, Y.: Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017). https://doi.org/10.1038/nnano.2017.16
Zheng, J.X., Tang, T., Zhao, Q., et al.: Physical orphaning versus chemical instability: is dendritic electrodeposition of Li fatal? ACS Energy Lett. 4, 1349–1355 (2019). https://doi.org/10.1021/acsenergylett.9b00750
Cheng, J.H., Assegie, A.A., Huang, C.J., et al.: Visualization of lithium plating and stripping via in operando transmission X-ray microscopy. J. Phys. Chem. C 121, 7761–7766 (2017). https://doi.org/10.1021/acs.jpcc.7b01414
Feng, X.N., Zheng, S.Q., Ren, D.S., et al.: Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 246, 53–64 (2019). https://doi.org/10.1016/j.apenergy.2019.04.009
Feng, X.N., Ren, D.S., He, X.M., et al.: Mitigating thermal runaway of lithium-ion batteries. Joule 4, 743–770 (2020). https://doi.org/10.1016/j.joule.2020.02.010
Ren, D.S., Feng, X.N., Lu, L.G., et al.: Overcharge behaviors and failure mechanism of lithium-ion batteries under different test conditions. Appl. Energy 250, 323–332 (2019). https://doi.org/10.1016/j.apenergy.2019.05.015
Niu, C., Liu, D., Lochala, J.A., et al.: Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy 6, 723–732 (2021). https://doi.org/10.1038/s41560-021-00852-3
Cao, W.Z., Zhang, J.N., Li, H.: Batteries with high theoretical energy densities. Energy Storage Mater. 26, 46–55 (2020). https://doi.org/10.1016/j.ensm.2019.12.024
Ye, Y.S., Chou, L.Y., Liu, Y.Y., et al.: Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat. Energy 5, 786–793 (2020). https://doi.org/10.1038/s41560-020-00702-8
Zhang, L.L., Zhao, X.S.: Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 38, 2520–2531 (2009). https://doi.org/10.1039/b813846j
Xiao, J.: How lithium dendrites form in liquid batteries. Science 366, 426–427 (2019). https://doi.org/10.1126/science.aay8672
Messer, R., Noack, F.: Nuclear magnetic relaxation by self-diffusion in solid lithium: T1-frequency dependence. Appl. Phys. 6, 79–88 (1975). https://doi.org/10.1007/BF00883553
Zou, P.C., Sui, Y.M., Zhan, H.C., et al.: Polymorph evolution mechanisms and regulation strategies of lithium metal anode under multiphysical fields. Chem. Rev. 121, 5986–6056 (2021). https://doi.org/10.1021/acs.chemrev.0c01100
Foroozan, T., Sharifi-Asl, S., Shahbazian-Yassar, R.: Mechanistic understanding of Li dendrites growth by in- situ/operando imaging techniques. J. Power Sources 461, 228135 (2020). https://doi.org/10.1016/j.jpowsour.2020.228135
Liu, J., Yuan, H., Cheng, X.B., et al.: A review of naturally derived nanostructured materials for safe lithium metal batteries. Mater. Today Nano 8, 100049 (2019). https://doi.org/10.1016/j.mtnano.2019.100049
Niu, C., Pan, H., Xu, W., et al.: Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 14, 594–601 (2019). https://doi.org/10.1038/s41565-019-0427-9
Gu, Y., Wang, W.W., Li, Y.J., et al.: Designable ultra-smooth ultra-thin solid-electrolyte interphases of three alkali metal anodes. Nat. Commun. 9, 1339 (2018). https://doi.org/10.1038/s41467-018-03466-8
Schaefer, J.L., Yanga, D.A., Archer, L.A.: High lithium transference number electrolytes via creation of 3-dimensional, charged, nanoporous networks from dense functionalized nanoparticle composites. Chem. Mater. 25, 834–839 (2013). https://doi.org/10.1021/cm303091j
Wang, A.X., Deng, Q.B., Deng, L.J., et al.: Eliminating tip dendrite growth by Lorentz force for stable lithium metal anodes. Adv. Funct. Mater. 29, 1902630 (2019). https://doi.org/10.1002/adfm.201902630
Tu, Z.Y., Zachman, M.J., Choudhury, S., et al.: Rechargeable batteries: nanoporous hybrid electrolytes for high-energy batteries based on reactive metal anodes. Adv. Energy Mater. 7, 1602367 (2017). https://doi.org/10.1002/aenm.201770039
Yu, Z.A., Cui, Y., Bao, Z.N.: Design principles of artificial solid electrolyte interphases for lithium-metal anodes. Cell Rep. Phys. Sci. 1, 100119 (2020). https://doi.org/10.1016/j.xcrp.2020.100119
Yamada, Y., Wang, J., Ko, S., et al.: Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019). https://doi.org/10.1038/s41560-019-0336-z
Lin, D.C., Yuen, P.Y., Liu, Y.Y., et al.: A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv. Mater. 30, 1802661 (2018). https://doi.org/10.1002/adma.201802661
Shen, X., Zhang, R., Shi, P., et al.: How does external pressure shape Li dendrites in Li metal batteries? Adv. Energy Mater. 11, 2003416 (2021). https://doi.org/10.1002/aenm.202003416
Wu, X., Zhang, W., Wu, N.Q., et al.: Structural evolution upon delithiation/lithiation in prelithiated foil anodes: a case study of AgLi alloys with high Li utilization and marginal volume variation. Adv. Energy Mater. 11, 2003082 (2021). https://doi.org/10.1002/aenm.202003082
Wang, H.S., Li, Y.Z., Li, Y.B., et al.: Wrinkled graphene cages as hosts for high-capacity Li metal anodes shown by cryogenic electron microscopy. Nano Lett. 19, 1326–1335 (2019). https://doi.org/10.1021/acs.nanolett.8b04906
Li, B.Q., Chen, X.R., Chen, X., et al.: Favorable lithium nucleation on lithiophilic framework porphyrin for dendrite-free lithium metal anodes. Research 2019, 4608940 (2019). https://doi.org/10.34133/2019/4608940
Wang, Z.S., Yu, J.W., Rao, M.M., et al.: Challenges, mitigation strategies and perspectives in development of Li metal anode. Nano Sel. 1, 622–638 (2020). https://doi.org/10.1002/nano.202000123
Liu, Y.Y., Xiong, S.Z., Wang, J.L., et al.: Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Storage Mater. 19, 24–30 (2019). https://doi.org/10.1016/j.ensm.2018.10.015
Pei, A., Zheng, G.Y., Shi, F.F., et al.: Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 17, 1132–1139 (2017). https://doi.org/10.1021/acs.nanolett.6b04755
Kashchiev, D.: On the relation between nucleation work, nucleus size, and nucleation rate. J. Chem. Phys. 76, 5098–5102 (1982). https://doi.org/10.1063/1.442808
Ely, D.R., García, R.E.: Heterogeneous nucleation and growth of lithium electrodeposits on negative electrodes. J. Electrochem. Soc. 160, A662–A668 (2013). https://doi.org/10.1149/1.057304jes
Wang, X., Pawar, G., Li, Y., et al.: Glassy Li metal anode for high-performance rechargeable Li batteries. Nat. Mater. 19, 1339–1345 (2020). https://doi.org/10.1038/s41563-020-0729-1
Yan, K., Lu, Z., Lee, H.W., et al.: Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 16010 (2016). https://doi.org/10.1038/nenergy.2016.10
Meng, Q.Q., Deng, B., Zhang, H.M., et al.: Heterogeneous nucleation and growth of electrodeposited lithium metal on the basal plane of single-layer graphene. Energy Storage Mater. 16, 419–425 (2019). https://doi.org/10.1016/j.ensm.2018.06.024
Tantratian, K., Cao, D.X., Abdelaziz, A., et al.: Stable Li metal anode enabled by space confinement and uniform curvature through lithiophilic nanotube arrays. Adv. Energy Mater. 10, 1902819 (2020). https://doi.org/10.1002/aenm.201902819
Jiang, Y., Wang, Z.X., Xu, C.X., et al.: Atomic layer deposition for improved lithiophilicity and solid electrolyte interface stability during lithium plating. Energy Storage Mater. 28, 17–26 (2020). https://doi.org/10.1016/j.ensm.2020.01.019
Kajikawa, Y., Noda, S.: Growth mode during initial stage of chemical vapor deposition. Appl. Surf. Sci. 245, 281–289 (2005). https://doi.org/10.1016/j.apsusc.2004.10.021
Gu, C.D., Zhang, T.Y.: Electrochemical synthesis of silver polyhedrons and dendritic films with superhydrophobic surfaces. Langmuir 24, 12010–12016 (2008). https://doi.org/10.1021/la802354n
Park, C.M., Kim, J.H., Kim, H., et al.: Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 39, 3115 (2010). https://doi.org/10.1039/b919877f
Wang, D., Zhang, W., Zheng, W.T., et al.: Towards high-safe lithium metal anodes: suppressing lithium dendrites via tuning surface energy. Adv. Sci. 4, 1600168 (2017). https://doi.org/10.1002/advs.201600168
Liu, X.H., Zhong, L., Zhang, L.Q., et al.: Lithium fiber growth on the anode in a nanowire lithium ion battery during charging. Appl. Phys. Lett. 98, 183107 (2011). https://doi.org/10.1063/1.3585655
Barton, J.L., Bockris, J.O.M.: The electrolytic growth of dendrites from ionic solutions. Proc. R. Soc. Lond. A 268, 485–505 (1962). https://doi.org/10.1098/rspa.1962.0154
Diggle, J.W., Despic, A.R., Bockris, J.O.M.: The mechanism of the dendritic electrocrystallization of zinc. J. Electrochem. Soc. 116, 1503–1514 (1969). https://doi.org/10.1149/1.2411588
Monroe, C., Newman, J.: Dendrite growth in lithium/polymer systems. J. Electrochem. Soc. 150, A1377–A1384 (2003). https://doi.org/10.1149/1.1606686
Harry, K.J., Hallinan, D.T., Parkinson, D.Y., et al.: Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 13, 69–73 (2014). https://doi.org/10.1038/nmat3793
Steiger, J., Kramer, D., Mönig, R.: Mechanisms of dendritic growth investigated by in situ light microscopy during electrodeposition and dissolution of lithium. J. Power Sources 261, 112–119 (2014). https://doi.org/10.1016/j.jpowsour.2014.03.029
Yamaki, J.I., Tobishima, S.I., Hayashi, K., et al.: A consideration of the morphology of electrochemically deposited lithium in an organic electrolyte. J. Power Sources 74, 219–227 (1998). https://doi.org/10.1016/S0378-7753(98)00067-6
Kushima, A., So, K.P., Su, C., et al.: Liquid cell transmission electron microscopy observation of lithium metal growth and dissolution: Root growth, dead lithium and lithium flotsams. Nano Energy 32, 271–279 (2017). https://doi.org/10.1016/j.nanoen.2016.12.001
Frenck, L., Sethi, G.K., Maslyn, J.A., et al.: Factors that control the formation of dendrites and other morphologies on lithium metal anodes. Front. Energy Res. 7, 115 (2019). https://doi.org/10.3389/fenrg.2019.00115
Li, Y.Z., Li, Y.B., Pei, A., et al.: Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017). https://doi.org/10.1126/science.aam6014
Zhang, L., Yang, T., Du, C., et al.: Lithium whisker growth and stress generation in an in situ atomic force microscope-environmental transmission electron microscope set-up. Nat. Nanotechnol. 15, 94–98 (2020). https://doi.org/10.1038/s41565-019-0604-x
He, Y., Ren, X., Xu, Y., et al.: Origin of lithium whisker formation and growth under stress. Nat. Nanotechnol. 14, 1042–1047 (2019). https://doi.org/10.1038/s41565-019-0558-z
Steiger, J., Richter, G., Wenk, M., et al.: Comparison of the growth of lithium filaments and dendrites under different conditions. Electrochem. Commun. 50, 11–14 (2015). https://doi.org/10.1016/j.elecom.2014.11.002
Wood, K.N., Noked, M., Dasgupta, N.P.: Lithium metal anodes: toward an improved understanding of coupled morphological, electrochemical, and mechanical behavior. ACS Energy Lett. 2, 664–672 (2017). https://doi.org/10.1021/acsenergylett.6b00650
Bai, P., Li, J., Brushett, F.R., et al.: Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 9, 3221–3229 (2016). https://doi.org/10.1039/c6ee01674j
Zhang, Y.Y., Heim, F.M., Song, N.N., et al.: New insights into mossy Li induced anode degradation and its formation mechanism in Li–S batteries. ACS Energy Lett. 2, 2696–2705 (2017). https://doi.org/10.1021/acsenergylett.7b00886
Shi, Y., Wan, J., Liu, G.X., et al.: Interfacial evolution of lithium dendrites and their solid electrolyte interphase shells of quasi-solid-state lithium-metal batteries. Angew. Chem. Int. Ed. 59, 18120–18125 (2020). https://doi.org/10.1002/anie.202001117
Bai, P., Guo, J.Z., Wang, M., et al.: Interactions between lithium growths and nanoporous ceramic separators. Joule 2, 2434–2449 (2018). https://doi.org/10.1016/j.joule.2018.08.018
Eastwood, D.S., Bayley, P.M., Chang, H.J., et al.: Three-dimensional characterization of electrodeposited lithium microstructures using synchrotron X-ray phase contrast imaging. Chem. Commun. 51, 266–268 (2015). https://doi.org/10.1039/c4cc03187c
Aryanfar, A., Brooks, D.J., Colussi, A.J., et al.: Quantifying the dependence of dead lithium losses on the cycling period in lithium metal batteries. Phys. Chem. Chem. Phys. 16, 24965–24970 (2014). https://doi.org/10.1039/c4cp03590a
Wang, Y.K., Dang, D.Y., Wang, M., et al.: Mechanical behavior of electroplated mossy lithium at room temperature studied by flat punch indentation. Appl. Phys. Lett. 115, 043903 (2019). https://doi.org/10.1063/1.5111150
Wang, Y.K., Dang, D.Y., Xiao, X.C., et al.: Structure and mechanical properties of electroplated mossy lithium: effects of current density and electrolyte. Energy Storage Mater. 26, 276–282 (2020). https://doi.org/10.1016/j.ensm.2020.01.004
Wood, K.N., Kazyak, E., Chadwick, A.F., et al.: Dendrites and pits: untangling the complex behavior of lithium metal anodes through operando video microscopy. ACS Central Sci. 2, 790–801 (2016). https://doi.org/10.1021/acscentsci.6b00260
Steiger, J., Kramer, D., Mönig, R.: Microscopic observations of the formation, growth and shrinkage of lithium moss during electrodeposition and dissolution. Electrochim. Acta 136, 529–536 (2014). https://doi.org/10.1016/j.electacta.2014.05.120
Sanchez, A.J., Kazyak, E., Chen, Y.X., et al.: Plan-view operando video microscopy of Li metal anodes: identifying the coupled relationships among nucleation, morphology, and reversibility. ACS Energy Lett. 5, 994–1004 (2020). https://doi.org/10.1021/acsenergylett.0c00215
Harrison, K.L., Zavadil, K.R., Hahn, N.T., et al.: Lithium self-discharge and its prevention: direct visualization through in situ electrochemical scanning transmission electron microscopy. ACS Nano 11, 11194–11205 (2017). https://doi.org/10.1021/acsnano.7b05513
Takeda, Y., Yamamoto, O., Imanishi, N.: Lithium dendrite formation on a lithium metal anode from liquid, polymer and solid electrolytes. Electrochemistry 84, 210–218 (2016). https://doi.org/10.5796/electrochemistry.84.210
Niu, W.X., Xu, G.B.: Crystallographic control of noble metal nanocrystals. Nano Today 6, 265–285 (2011). https://doi.org/10.1016/j.nantod.2011.04.006
Wiley, B., Sun, Y.G., Mayers, B., et al.: Shape-controlled synthesis of metal nanostructures: the case of silver. Chem. A Eur. J. 11, 454–463 (2005). https://doi.org/10.1002/chem.200590003
Murphy, C.J., Sau, T.K., Gole, A.M., et al.: Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J. Phys. Chem. B 109, 13857–13870 (2005). https://doi.org/10.1021/jp0516846
Pande, V., Viswanathan, V.: Computational screening of current collectors for enabling anode-free lithium metal batteries. ACS Energy Lett. 4, 2952–2959 (2019). https://doi.org/10.1021/acsenergylett.9b02306
Hummelshøj, J.S., Luntz, A.C., Nørskov, J.K.: Theoretical evidence for low kinetic overpotentials in Li-O2 electrochemistry. J. Chem. Phys. 138, 034703 (2013). https://doi.org/10.1063/1.4773242
Zheng, J., Bock, D.C., Tang, T., et al.: Regulating electrodeposition morphology in high-capacity aluminium and zinc battery anodes using interfacial metal-substrate bonding. Nat. Energy 6, 398–406 (2021). https://doi.org/10.1038/s41560-021-00797-7
Cogswell, D.A.: Quantitative phase-field modeling of dendritic electrodeposition. Phys. Rev. E 92, 011301 (2015). https://doi.org/10.1103/physreve.92.011301
Sato, R.: Crystal growth of electrodeposited zinc. J. Electrochem. Soc. 106, 206–211 (1959). https://doi.org/10.1149/1.2427309
Perveen, S., Naqvi, I., Muhammad, M., et al.: Fractal growth of zinc dendrites. Asian J. Chem. 21, 4190–4198 (2009)
Faust, J.W., Jr.: Effect of electrodeposition parameters on growth habit and morphology. J. Cryst. Growth 3(4), 433–435 (1968). https://doi.org/10.1016/0022-0248(68)90193-0
Stark, J.K., Ding, Y., Kohl, P.A.: Nucleation of electrodeposited lithium metal: dendritic growth and the effect of co-deposited sodium. J. Electrochem. Soc. 160, D337–D342 (2013). https://doi.org/10.1149/2.028309jes
Nagy, K.S., Kazemiabnavi, S., Thornton, K., et al.: Thermodynamic overpotentials and nucleation rates for electrodeposition on metal anodes. ACS Appl. Mater. Interfaces 11, 7954–7964 (2019). https://doi.org/10.1021/acsami.8b19787
Wang, J., Wang, S.Q.: Surface energy and work function of fcc and bcc crystals: density functional study. Surf. Sci. 630, 216–224 (2014). https://doi.org/10.1016/j.susc.2014.08.017
Kokko, K., Salo, P.T., Laihia, R., et al.: First-principles calculations for work function and surface energy of thin lithium films. Surf. Sci. 348, 168–174 (1996). https://doi.org/10.1016/0039-6028(95)01029-7
Gaissmaier, D., Fantauzzi, D., Jacob, T.: First principles studies of self-diffusion processes on metallic lithium surfaces. J. Chem. Phys. 150, 041723 (2018). https://doi.org/10.1063/1.5056226
Zhang, X.L., Wang, W.K., Wang, A.B., et al.: Improved cycle stability and high security of Li-B alloy anode for lithium–sulfur battery. J. Mater. Chem. A 2, 11660–11665 (2014). https://doi.org/10.1039/c4ta01709a
Zu, C.X., Manthiram, A.: Stabilized lithium–metal surface in a polysulfide-rich environment of lithium–sulfur batteries. J. Phys. Chem. Lett. 5, 2522–2527 (2014). https://doi.org/10.1021/jz501352e
Hull, C.M., Switzer, J.A.: Electrodeposited epitaxial Cu(100) on Si(100) and lift-off of single crystal-like Cu(100) foils. ACS Appl. Mater. Interfaces 10, 38596–38602 (2018). https://doi.org/10.1021/acsami.8b13188
Allongue, P., Maroun, F.: Metal electrodeposition on single crystal metal surfaces mechanisms, structure and applications. Curr. Opin. Solid State Mater. Sci. 10, 173–181 (2006). https://doi.org/10.1016/j.cossms.2007.04.001
Petermann, J., Broza, G.: Epitaxial deposition of metals on uniaxial oriented semi-crystalline polymers. J. Mater. Sci. 22, 1108–1112 (1987). https://doi.org/10.1007/BF01103557
Zheng, J.X., Zhao, Q., Tang, T., et al.: Reversible epitaxial electrodeposition of metals in battery anodes. Science 366, 645–648 (2019). https://doi.org/10.1126/science.aax6873
Li, N., Zhang, K., Xie, K.Y., et al.: Reduced-graphene-oxide-guided directional growth of planar lithium layers. Adv. Mater. 32, 1907079 (2020). https://doi.org/10.1002/adma.201907079
Gu, Y., Xu, H.Y., Zhang, X.G., et al.: Lithiophilic faceted Cu(100) surfaces: high utilization of host surface and cavities for lithium metal anodes. Angew. Chem. Int. Ed. 131, 3124–3128 (2019). https://doi.org/10.1002/ange.201812523
Matsui, M.: Study on electrochemically deposited Mg metal. J. Power Sources 196, 7048–7055 (2011). https://doi.org/10.1016/j.jpowsour.2010.11.141
Ling, C., Banerjee, D., Matsui, M.: Study of the electrochemical deposition of Mg in the atomic level: why it prefers the non-dendritic morphology. Electrochim. Acta 76, 270–274 (2012). https://doi.org/10.1016/j.electacta.2012.05.001
Brune, H.: Microscopic view of epitaxial metal growth: nucleation and aggregation. Surf. Sci. Rep. 31, 125–229 (1998). https://doi.org/10.1016/S0167-5729(99)80001-6
Quayum, M.E., Ye, S., Uosaki, K.: Mechanism for nucleation and growth of electrochemical palladium deposition on an Au(111) electrode. J. Electroanal. Chem. 520, 126–132 (2002). https://doi.org/10.1016/S0022-0728(02)00643-5
Jäckle, M., Groß, A.: Microscopic properties of lithium, sodium, and magnesium battery anode materials related to possible dendrite growth. J. Chem. Phys. 141, 174710 (2014). https://doi.org/10.1063/1.4901055
Jäckle, M., Helmbrecht, K., Smits, M., et al.: Self-diffusion barriers: possible descriptors for dendrite growth in batteries? Energy Environ. Sci. 11, 3400–3407 (2018). https://doi.org/10.1039/c8ee01448e
Galdikas, A.: The influence of surface diffusion on surface roughness and component distribution profiles during deposition of multilayers. Comput. Mater. Sci. 38, 716–721 (2007). https://doi.org/10.1016/j.commatsci.2006.05.006
Wei, S.Y., Choudhury, S., Tu, Z.Y., et al.: Electrochemical interphases for high-energy storage using reactive metal anodes. Acc. Chem. Res. 51, 80–88 (2018). https://doi.org/10.1021/acs.accounts.7b00484
Shi, F.F., Pei, A., Vailionis, A., et al.: Strong texturing of lithium metal in batteries. P. Natl. Acad. Sci. U. S. A. 114, 12138–12143 (2017). https://doi.org/10.1073/pnas.1708224114
Chen, X.R., Yao, Y.X., Yan, C., et al.: A diffusion: reaction competition mechanism to tailor lithium deposition for lithium-metal batteries. Angew. Chem. Int. Ed. 59, 7743–7747 (2020). https://doi.org/10.1002/anie.202000375
LePage, W.S., Chen, Y.X., Kazyak, E., et al.: Lithium mechanics: roles of strain rate and temperature and implications for lithium metal batteries. J. Electrochem. Soc. 166, A89–A97 (2019). https://doi.org/10.1149/2.0221902jes
Wang, X., Zeng, W., Hong, L., et al.: Stress-driven lithium dendrite growth mechanism and dendrite mitigation by electroplating on soft substrates. Nat. Energy 3, 227–235 (2018). https://doi.org/10.1038/s41560-018-0104-5
Jana, A., Woo, S.I., Vikrant, K.S.N., et al.: Electrochemomechanics of lithium dendrite growth. Energy Environ. Sci. 12, 3595–3607 (2019). https://doi.org/10.1039/c9ee01864f
Genovese, M., Louli, A.J., Weber, R., et al.: Hot formation for improved low temperature cycling of anode-free lithium metal batteries. J. Electrochem. Soc. 166, A3342–A3347 (2019). https://doi.org/10.1149/2.0661914jes
Yin, X.S., Tang, W., Jung, I.D., et al.: Insights into morphological evolution and cycling behaviour of lithium metal anode under mechanical pressure. Nano Energy 50, 659–664 (2018). https://doi.org/10.1016/j.nanoen.2018.06.003
Xu, C., Ahmad, Z., Aryanfar, A., et al.: Enhanced strength and temperature dependence of mechanical properties of Li at small scales and its implications for Li metal anodes. P. Natl. Acad. Sci. U. S. A. 114, 57–61 (2017). https://doi.org/10.1073/pnas.1615733114
Greer, J.R., Weinberger, C.R., Cai, W.: Comparing the strength of f.c.c. and b.c.c. sub-micrometer pillars: compression experiments and dislocation dynamics simulations. Mater. Sci. Eng. A 493, 21–25 (2008). https://doi.org/10.1016/j.msea.2007.08.093
Weinberger, C.R., Cai, W.: Surface-controlled dislocation multiplication in metal micropillars. P. Natl. Acad. Sci. U. S. A. 105, 14304–14307 (2008). https://doi.org/10.1073/pnas.0806118105
Tang, Y.F., Zhang, L.Q., Chen, J.Z., et al.: Electro-chemo-mechanics of lithium in solid state lithium metal batteries. Energy Environ. Sci. 14, 602–642 (2021). https://doi.org/10.1039/d0ee02525a
Bruce, P.G., Vincent, C.A.: Steady state current flow in solid binary electrolyte cells. J. Electroanal. Chem. Interfacial Electrochem. 225, 1–17 (1987). https://doi.org/10.1016/0022-0728(87)80001-3
Rosso, M., Brissot, C., Teyssot, A., et al.: Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 51, 5334–5340 (2006). https://doi.org/10.1016/j.electacta.2006.02.004
Rosso, M., Gobron, T., Brissot, C., et al.: Onset of dendritic growth in lithium/polymer cells. J. Power Sources 97(98), 804–806 (2001). https://doi.org/10.1016/S0378-7753(01)00734-0
Chazalviel, J.N.: Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 42, 7355–7367 (1990). https://doi.org/10.1103/physreva.42.7355
Brissot, C., Rosso, M., Chazalviel, J.N., et al.: Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 81(82), 925–929 (1999). https://doi.org/10.1016/S0378-7753(98)00242-0
Lee, Y., Ma, B.Y., Bai, P.: Concentration polarization and metal dendrite initiation in isolated electrolyte microchannels. Energy Environ. Sci. 13, 3504–3513 (2020). https://doi.org/10.1039/d0ee01874k
Chang, H.J., Ilott, A.J., Trease, N.M., et al.: Correlating microstructural lithium metal growth with electrolyte salt depletion in lithium batteries using 7Li MRI. J. Am. Chem. Soc. 137, 15209–15216 (2015). https://doi.org/10.1021/jacs.5b09385
Cheng, Q., Wei, L., Liu, Z., et al.: Operando and three-dimensional visualization of anion depletion and lithium growth by stimulated raman scattering microscopy. Nat. Commun. 9, 2942 (2018). https://doi.org/10.1038/s41467-018-05289-z
Freudiger, C.W., Min, W., Saar, B.G., et al.: Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008). https://doi.org/10.1126/science.1165758
Zheng, J.X., Kim, M.S., Tu, Z.Y., et al.: Regulating electrodeposition morphology of lithium: towards commercially relevant secondary Li metal batteries. Chem. Soc. Rev. 49, 2701–2750 (2020). https://doi.org/10.1039/c9cs00883g
Tikekar, M.D., Archer, L.A., Koch, D.L.: Stability analysis of electrodeposition across a structured electrolyte with immobilized anions. J. Electrochem. Soc. 161, A847–A855 (2014). https://doi.org/10.1149/2.085405jes
Tikekar, M.D., Choudhury, S., Tu, Z., et al.: Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114 (2016). https://doi.org/10.1038/nenergy.2016.114
Fleury, V., Kaufman, J., Hibbert, B.: Evolution of the space-charge layer during electrochemical deposition with convection. Phys. Rev. E 48, 3831–3840 (1993). https://doi.org/10.1103/physreve.48.3831
Rubinstein, I., Zaltzman, B.: Electro-osmotically induced convection at a permselective membrane. Phys. Rev. E 62, 2238–2251 (2000). https://doi.org/10.1103/physreve.62.2238
Fleury, V., Kaufman, J.H., Hibbert, D.B.: Mechanism of a morphology transition in ramified electrochemical growth. Nature 367, 435–438 (1994). https://doi.org/10.1038/367435a0
Tikekar, M.D., Li, G.J., Archer, L.A., et al.: Electroconvection and morphological instabilities in potentiostatic electrodeposition across liquid electrolytes with polymer additives. J. Electrochem. Soc. 165, A3697–A3713 (2018). https://doi.org/10.1149/2.0271816jes
Wei, S.Y., Cheng, Z., Nath, P., et al.: Stabilizing electrochemical interfaces in viscoelastic liquid electrolytes. Sci. Adv. 4, eaao6243 (2018). https://doi.org/10.1126/sciadv.aao6243
Warren, A., Zhang, D.H., Choudhury, S., et al.: Electrokinetics in viscoelastic liquid electrolytes above the diffusion limit. Macromolecules 52, 4666–4672 (2019). https://doi.org/10.1021/acs.macromol.9b00536
Maletzki, F., Rösler, H.W., Staude, E.: Ion transfer across electrodialysis membranes in the overlimiting current range: stationary voltage current characteristics and current noise power spectra under different conditions of free convection. J. Membr. Sci. 71, 105–116 (1992). https://doi.org/10.1016/0376-7388(92)85010-G
Khurana, R., Schaefer, J.L., Archer, L.A., et al.: Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014). https://doi.org/10.1021/ja502133j
Goodenough, J.B., Kim, Y.: Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010). https://doi.org/10.1021/cm901452z
Tikekar, M.D., Archer, L.A., Koch, D.L.: Stabilizing electrodeposition in elastic solid electrolytes containing immobilized anions. Sci. Adv. 2, e1600320 (2016). https://doi.org/10.1126/sciadv.1600320
Lu, Y., Tu, Z., Archer, L.A.: Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014). https://doi.org/10.1038/nmat4041
He, X., Bresser, D., Passerini, S., et al.: The passivity of lithium electrodes in liquid electrolytes for secondary batteries. Nat. Rev. Mater. 6, 1036–1052 (2021). https://doi.org/10.1038/s41578-021-00345-5
Agubra, V.A., Fergus, J.W.: The formation and stability of the solid electrolyte interface on the graphite anode. J. Power Sources 268, 153–162 (2014). https://doi.org/10.1016/j.jpowsour.2014.06.024
Yan, C., Xu, R., Xiao, Y., et al.: Toward critical electrode/electrolyte interfaces in rechargeable batteries. Adv. Funct. Mater. 30, 1909887 (2020). https://doi.org/10.1002/adfm.201909887
Weadock, N., Varongchayakul, N., Wan, J.Y., et al.: Determination of mechanical properties of the SEI in sodium ion batteries via colloidal probe microscopy. Nano Energy 2, 713–719 (2013). https://doi.org/10.1016/j.nanoen.2013.08.005
Verma, P., Maire, P., Novák, P.: A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 55, 6332–6341 (2010). https://doi.org/10.1016/j.electacta.2010.05.072
Zhai, P.B., Liu, L.X., Gu, X.K., et al.: Interface engineering for lithium metal anodes in liquid electrolyte. Adv. Energy Mater. 10, 2001257 (2020). https://doi.org/10.1002/aenm.202001257
Cheng, X.B., Zhang, R., Zhao, C.Z., et al.: A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 3, 1500213 (2016). https://doi.org/10.1002/advs.201500213
Moumouzias, G., Ritzoulis, G., Siapkas, D., et al.: Comparative study of LiBF4, LiAsF6, LiPF6, and LiClO4 as electrolytes in propylene carbonate-diethyl carbonate solutions for Li/LiMn2O4 cells. J. Power Sources 122, 57–66 (2003). https://doi.org/10.1016/S0378-7753(03)00348-3
Logan, E.R., Tonita, E.M., Gering, K.L., et al.: A critical evaluation of the advanced electrolyte model. J. Electrochem. Soc. 165, A3350–A3359 (2018). https://doi.org/10.1149/2.0471814jes
Arnaud, R., Benrabah, D., Sanchez, J.Y.: Theoretical study of CF3SO3Li, (CF3SO2)2NLi, and (CF3SO2)2CHLi ion pairs. J. Phys. Chem. 100, 10882–10891 (1996). https://doi.org/10.1021/jp953259q
Chen, R.J., Wu, F., Xu, B., et al.: Binary complex electrolytes based on LiX[X=N(SO2CF3)2–, CF3SO3–, ClO4–]-acetamide for electric double layer capacitors. J. Electrochem. Soc. 154, A703–A708 (2007). https://doi.org/10.1149/1.2737350
Tu, Z.Y., Nath, P., Lu, Y.Y., et al.: Nanostructured electrolytes for stable lithium electrodeposition in secondary batteries. Acc. Chem. Res. 48, 2947–2956 (2015). https://doi.org/10.1021/acs.accounts.5b00427
An, S.J., Li, J.L., Daniel, C., et al.: The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 105, 52–76 (2016). https://doi.org/10.1016/j.carbon.2016.04.008
Li, L.L., Li, S.Y., Lu, Y.Y.: Suppression of dendritic lithium growth in lithium metal-based batteries. Chem. Commun. 54, 6648–6661 (2018). https://doi.org/10.1039/c8cc02280a
Zhang, X.Q., Chen, X., Cheng, X.B., et al.: Highly stable lithium metal batteries enabled by regulating the solvation of lithium ions in nonaqueous electrolytes. Angew. Chem. Int. Ed. 57, 5301–5305 (2018). https://doi.org/10.1002/anie.201801513
Zhang, X.Q., Cheng, X.B., Chen, X., et al.: Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv. Funct. Mater. 27, 1605989 (2017). https://doi.org/10.1002/adfm.201605989
Chen, X., Zhang, Q.: Atomic insights into the fundamental interactions in lithium battery electrolytes. Acc. Chem. Res. 53, 1992–2002 (2020). https://doi.org/10.1021/acs.accounts.0c00412
Magnussen, O.M., Groß, A.: Toward an atomic-scale understanding of electrochemical interface structure and dynamics. J. Am. Chem. Soc. 141, 4777–4790 (2019). https://doi.org/10.1021/jacs.8b13188
Yan, C., Yao, Y.X., Chen, X., et al.: Lithium nitrate solvation chemistry in carbonate electrolyte sustains high-voltage lithium metal batteries. Angew. Chem. Int. Ed. 57, 14055–14059 (2018). https://doi.org/10.1002/anie.201807034
Yan, C., Li, H.R., Chen, X., et al.: Regulating the inner Helmholtz plane for stable solid electrolyte interphase on lithium metal anodes. J. Am. Chem. Soc. 141, 9422–9429 (2019). https://doi.org/10.1021/jacs.9b05029
Delp, S.A., Borodin, O., Olguin, M., et al.: Importance of reduction and oxidation stability of high voltage electrolytes and additives. Electrochim. Acta 209, 498–510 (2016). https://doi.org/10.1016/j.electacta.2016.05.100
Garreau, M.: Cyclability of the lithium electrode. J. Power Sources 20, 9–17 (1987). https://doi.org/10.1016/0378-7753(87)80085-X
Warren, L.F., Anderson, D.P.: Polypyrrole films from aqueous electrolytes: the effect of anions upon order. J. Electrochem. Soc. 134, 101–105 (1987). https://doi.org/10.1149/1.2100383
Peled, E., Golodnitsky, D., Ardel, G.: Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 144, L208–L210 (1997). https://doi.org/10.1149/1.1837858
Xu, K.: Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014). https://doi.org/10.1021/cr500003w
Benitez, L., Seminario, J.M.: Electron transport and electrolyte reduction in the solid-electrolyte interphase of rechargeable lithium ion batteries with silicon anodes. J. Phys. Chem. C 120, 17978–17988 (2016). https://doi.org/10.1021/acs.jpcc.6b06446
Hobold, G.M."., Lopez, J., Guo, R., et al.: Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951–960 (2021). https://doi.org/10.1038/s41560-021-00910-w
Liu, Z., Qi, Y., Lin, Y.X., et al.: Interfacial study on solid electrolyte interphase at Li metal anode: implication for Li dendrite growth. J. Electrochem. Soc. 163, A592–A598 (2016). https://doi.org/10.1149/2.0151605jes
Lin, Y.X., Liu, Z., Leung, K., et al.: Connecting the irreversible capacity loss in Li-ion batteries with the electronic insulating properties of solid electrolyte interphase (SEI) components. J. Power Sources 309, 221–230 (2016). https://doi.org/10.1016/j.jpowsour.2016.01.078
Fang, C., Li, J., Zhang, M., et al.: Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019). https://doi.org/10.1038/s41586-019-1481-z
Shi, S.Q., Lu, P., Liu, Z.Y., et al.: Direct calculation of Li-ion transport in the solid electrolyte interphase. J. Am. Chem. Soc. 134, 15476–15487 (2012). https://doi.org/10.1021/ja305366r
Ramasubramanian, A., Yurkiv, V., Foroozan, T., et al.: Lithium diffusion mechanism through solid–electrolyte interphase in rechargeable lithium batteries. J. Phys. Chem. C 123, 10237–10245 (2019). https://doi.org/10.1021/acs.jpcc.9b00436
Piao, N., Liu, S.F., Zhang, B., et al.: Lithium metal batteries enabled by synergetic additives in commercial carbonate electrolytes. ACS Energy Lett. 6, 1839–1848 (2021). https://doi.org/10.1021/acsenergylett.1c00365
Li, Y.Z., Huang, W., Li, Y.B., et al.: Correlating structure and function of battery interphases at atomic resolution using cryoelectron microscopy. Joule 2, 2167–2177 (2018). https://doi.org/10.1016/j.joule.2018.08.004
Aurbach, D.: Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 89, 206–218 (2000). https://doi.org/10.1016/S0378-7753(00)00431-6
Wang, T., Salvatierra, R.V., Tour, J.M.: Detecting Li dendrites in a two-electrode battery system. Adv. Mater. 31, 1807405 (2019). https://doi.org/10.1002/adma.201807405
Liu, H., Wang, E.R., Zhang, Q., et al.: Unique 3D nanoporous/macroporous structure Cu current collector for dendrite-free lithium deposition. Energy Storage Mater. 17, 253–259 (2019). https://doi.org/10.1016/j.ensm.2018.07.010
Ma, X.T., Liu, Z.T., Chen, H.L.: Facile and scalable electrodeposition of copper current collectors for high-performance Li-metal batteries. Nano Energy 59, 500–507 (2019). https://doi.org/10.1016/j.nanoen.2019.02.048
Yue, X.Y., Wang, W.W., Wang, Q.C., et al.: Cuprite-coated Cu foam skeleton host enabling lateral growth of lithium dendrites for advanced Li metal batteries. Energy Storage Mater. 21, 180–189 (2019). https://doi.org/10.1016/j.ensm.2018.12.00
Adair, K.R., Iqbal, M., Wang, C.H., et al.: Towards high performance Li metal batteries: nanoscale surface modification of 3D metal hosts for pre-stored Li metal anodes. Nano Energy 54, 375–382 (2018). https://doi.org/10.1016/j.nanoen.2018.10.002
Huang, K., Li, Z., Xu, Q.J., et al.: Lithiophilic CuO nanoflowers on Ti-mesh inducing lithium lateral plating enabling stable lithium-metal anodes with ultrahigh rates and ultralong cycle life. Adv. Energy Mater. 9, 1900853 (2019). https://doi.org/10.1002/aenm.201900853
Zhu, J.F., Chen, J., Luo, Y., et al.: Lithiophilic metallic nitrides modified nickel foam by plasma for stable lithium metal anode. Energy Storage Mater. 23, 539–546 (2019). https://doi.org/10.1016/j.ensm.2019.04.005
Pu, J., Li, J., Zhang, K., et al.: Conductivity and lithiophilicity gradients guide lithium deposition to mitigate short circuits. Nat. Commun. 10, 1896 (2019). https://doi.org/10.1038/s41467-019-09932-1
Li, J., Zou, P.C., Chiang, S.W., et al.: A conductive-dielectric gradient framework for stable lithium metal anode. Energy Storage Mater. 24, 700–706 (2020). https://doi.org/10.1016/j.ensm.2019.06.019
Cheng, Y.F., Ke, X., Chen, Y.M., et al.: Lithiophobic-lithiophilic composite architecture through co-deposition technology toward high-performance lithium metal batteries. Nano Energy 63, 103854 (2019). https://doi.org/10.1016/j.nanoen.2019.103854
Lin, D., Liu, Y., Liang, Z., et al.: Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 11, 626–632 (2016). https://doi.org/10.1038/nnano.2016.32
Xu, Y., Li, T., Wang, L.P., et al.: Interlayered dendrite-free lithium plating for high-performance lithium-metal batteries. Adv. Mater. 31, 1901662 (2019). https://doi.org/10.1002/adma.201901662
Zheng, J.X., Zhao, Q., Liu, X., et al.: Nonplanar electrode architectures for ultrahigh areal capacity batteries. ACS Energy Lett. 4, 271–275 (2019). https://doi.org/10.1021/acsenergylett.8b02131
Chen, Y.Z., Elangovan, A., Zeng, D.L., et al.: Vertically aligned carbon nanofibers on Cu foil as a 3D current collector for reversible Li plating/stripping toward high-performance Li–S batteries. Adv. Funct. Mater. 30, 1906444 (2020). https://doi.org/10.1002/adfm.201906444
Huang, G.X., Chen, S.R., Guo, P.M., et al.: In situ constructing lithiophilic NiFx nanosheets on Ni foam current collector for stable lithium metal anode via a succinct fluorination strategy. Chem. Eng. J. 395, 125122 (2020). https://doi.org/10.1016/j.cej.2020.125122
Zheng, H.F., Zhang, Q.F., Chen, Q.L., et al.: 3D lithiophilic–lithiophobic–lithiophilic dual-gradient porous skeleton for highly stable lithium metal anode. J. Mater. Chem. A 8, 313–322 (2020). https://doi.org/10.1039/c9ta09505e
Zhao, Y., Hao, S.G., Su, L., et al.: Hierarchical Cu fibers induced Li uniform nucleation for dendrite-free lithium metal anode. Chem. Eng. J. 392, 123691 (2020). https://doi.org/10.1016/j.cej.2019.123691
Chen, K.H., Sanchez, A.J., Kazyak, E., et al.: Synergistic effect of 3D current collectors and ALD surface modification for high coulombic efficiency lithium metal anodes. Adv. Energy Mater. 9, 1802534 (2019). https://doi.org/10.1002/aenm.201802534
Zhang, C., Lyu, R.Y., Lv, W., et al.: A lightweight 3D Cu nanowire network with phosphidation gradient as current collector for high-density nucleation and stable deposition of lithium. Adv. Mater. 31, 1904991 (2019). https://doi.org/10.1002/adma.201904991
Pei, F., Fu, A., Ye, W.B., et al.: Robust lithium metal anodes realized by lithiophilic 3D porous current collectors for constructing high-energy lithium–sulfur batteries. ACS Nano 13, 8337–8346 (2019). https://doi.org/10.1021/acsnano.9b03784
Ke, X., Liang, Y.H., Ou, L.H., et al.: Surface engineering of commercial Ni foams for stable Li metal anodes. Energy Storage Mater. 23, 547–555 (2019). https://doi.org/10.1016/j.ensm.2019.04.003
Yang, G.H., Chen, J.D., Xiao, P.T., et al.: Graphene anchored on Cu foam as a lithiophilic 3D current collector for a stable and dendrite-free lithium metal anode. J. Mater. Chem. A 6, 9899–9905 (2018). https://doi.org/10.1039/c8ta02810a
Zuo, T.T., Wu, X.W., Yang, C.P., et al.: Graphitized carbon fibers as multifunctional 3D current collectors for high areal capacity Li anodes. Adv. Mater. 29, 1700389 (2017). https://doi.org/10.1002/adma.201700389
Zhang, R., Wen, S.W., Wang, N., et al.: N-doped graphene modified 3D porous Cu current collector toward microscale homogeneous Li deposition for Li metal anodes. Adv. Energy Mater. 8, 1800914 (2018). https://doi.org/10.1002/aenm.201800914
Yan, K., Sun, B., Munroe, P., et al.: Three-dimensional pie-like current collectors for dendrite-free lithium metal anodes. Energy Storage Mater. 11, 127–133 (2018). https://doi.org/10.1016/j.ensm.2017.10.012
Hou, Z., Yu, Y.K., Wang, W.H., et al.: Lithiophilic Ag nanoparticle layer on Cu current collector toward stable Li metal anode. ACS Appl. Mater. Interfaces 11, 8148–8154 (2019). https://doi.org/10.1021/acsami.9b01521
Huang, Z.J., Zhang, C., Lv, W., et al.: Realizing stable lithium deposition by in situ grown Cu2S nanowires inside commercial Cu foam for lithium metal anodes. J. Mater. Chem. A 7, 727–732 (2019). https://doi.org/10.1039/c8ta10341k
Liu, L., Yin, Y.X., Li, J.Y., et al.: Free-standing hollow carbon fibers as high-capacity containers for stable lithium metal anodes. Joule 1, 563–575 (2017). https://doi.org/10.1016/j.joule.2017.06.004
He, Y., Xu, H.W., Shi, J.L., et al.: Polydopamine coating layer modified current collector for dendrite-free Li metal anode. Energy Storage Mater. 23, 418–426 (2019). https://doi.org/10.1016/j.ensm.2019.04.026
Wang, S.H., Yin, Y.X., Zuo, T.T., et al.: Stable Li metal anodes via regulating lithium plating/stripping in vertically aligned microchannels. Adv. Mater. 29, 1703729 (2017). https://doi.org/10.1002/adma.201703729
Lin, K., Qin, X.Y., Liu, M., et al.: Ultrafine titanium nitride sheath decorated carbon nanofiber network enabling stable lithium metal anodes. Adv. Funct. Mater. 29, 1903229 (2019). https://doi.org/10.1002/adfm.201903229
Zhang, R., Wang, N., Shi, C.S., et al.: Spatially uniform Li deposition realized by 3D continuous duct-like graphene host for high energy density Li metal anode. Carbon 161, 198–205 (2020). https://doi.org/10.1016/j.carbon.2020.01.077
Huang, S.B., Yang, H., Hu, J.K., et al.: Early lithium plating behavior in confined nanospace of 3D lithiophilic carbon matrix for stable solid-state lithium metal batteries. Small 15, 1904216 (2019). https://doi.org/10.1002/smll.201904216
Zhang, D., Dai, A., Fan, B.F., et al.: Three-dimensional ordered macro/mesoporous Cu/Zn as a lithiophilic current collector for dendrite-free lithium metal anode. ACS Appl. Mater. Interfaces 12, 31542–31551 (2020). https://doi.org/10.1021/acsami.0c09503
Cao, J.Q., Deng, L.Y., Wang, X.H., et al.: Stable lithium metal anode achieved by in situ grown CuO nanowire arrays on Cu foam. Energy Fuels 34, 7684–7691 (2020). https://doi.org/10.1021/acs.energyfuels.0c01180
Huang, S., Zhang, W., Ming, H., et al.: Chemical energy release driven lithiophilic layer on 1 m2 commercial brass mesh toward highly stable lithium metal batteries. Nano Lett. 19, 1832–1837 (2019). https://doi.org/10.1021/acs.nanolett.8b04919
Zhang, Z., Wang, J.L., Yan, X.F., et al.: In-situ growth of hierarchical N-doped CNTs/Ni foam scaffold for dendrite-free lithium metal anode. Energy Storage Mater. 29, 332–340 (2020). https://doi.org/10.1016/j.ensm.2020.04.022
Liu, Y., Lin, D., Liang, Z., et al.: Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 7, 10992 (2016). https://doi.org/10.1038/ncomms10992
Liang, Z., Lin, D.C., Zhao, J., et al.: Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. P. Natl. Acad. Sci. U. S. A. 113, 2862–2867 (2016). https://doi.org/10.1073/pnas.1518188113
Zhang, P.C., Peng, C.X., Liu, X.S., et al.: 3D lithiophilic “hairy” Si nanowire arrays @ carbon scaffold favor a flexible and stable lithium composite anode. ACS Appl. Mater. Interfaces 11, 44325–44332 (2019). https://doi.org/10.1021/acsami.9b15250
Liang, Z., Lin, D.C., Zhao, J., et al.: Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Nat. Acad. Sci. U. S. A. 113, 2862–2867 (2016). https://doi.org/10.1073/pnas.1518188113
Liu, T.C., Chen, S.Q., Sun, W.W., et al.: Lithiophilic vertical cactus-like framework derived from Cu/Zn-based coordination polymer through in situ chemical etching for stable lithium metal batteries. Adv. Funct. Mater. 31, 2008514 (2021). https://doi.org/10.1002/adfm.202008514
Yue, X.Y., Wang, W.W., Wang, Q.C., et al.: CoO nanofiber decorated nickel foams as lithium dendrite suppressing host skeletons for high energy lithium metal batteries. Energy Storage Mater. 14, 335–344 (2018). https://doi.org/10.1016/j.ensm.2018.05.017
Chen, L., Chen, G., Tang, W., et al.: A robust and lithiophilic three-dimension framework of CoO nanorod arrays on carbon cloth for cycling-stable lithium metal anodes. Mater. Today Energy 18, 100520 (2020). https://doi.org/10.1016/j.mtener.2020.100520
Yue, X.Y., Li, X.L., Wang, W.W., et al.: Wettable carbon felt framework for high loading Li-metal composite anode. Nano Energy 60, 257–266 (2019). https://doi.org/10.1016/j.nanoen.2019.03.057
Wu, S.L., Su, B.Z., Jiang, H., et al.: Lithiophilicity conversion of carbon paper with uniform Cu2+1O coating: boosting stable Li-Cu2+1O-CP composite anode through melting infusion. Chem. Eng. J. 388, 124238 (2020). https://doi.org/10.1016/j.cej.2020.124238
Wang, S.H., Yue, J.P., Dong, W., et al.: Tuning wettability of molten lithium via a chemical strategy for lithium metal anodes. Nat. Commun. 10, 4930 (2019). https://doi.org/10.1038/s41467-019-12938-4
Zheng, Z.J., Ye, H., Guo, Z.P.: Recent progress in designing stable composite lithium anodes with improved wettability. Adv. Sci. 7, 2002212 (2020). https://doi.org/10.1002/advs.202002212
Li, Q., Zhu, S.P., Lu, Y.Y.: 3D porous Cu current collector/Li-metal composite anode for stable lithium-metal batteries. Adv. Funct. Mater. 27, 1606422 (2017). https://doi.org/10.1002/adfm.201606422
Yang, C.P., Yin, Y.X., Zhang, S.F., et al.: Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 6, 8058 (2015). https://doi.org/10.1038/ncomms9058
Chen, H., Pei, A., Wan, J.Y., et al.: Tortuosity effects in lithium-metal host anodes. Joule 4, 938–952 (2020). https://doi.org/10.1016/j.joule.2020.03.008
Tang, W., Yin, X.S., Chen, Z.X., et al.: Chemically polished lithium metal anode for high energy lithium metal batteries. Energy Storage Mater. 14, 289–296 (2018). https://doi.org/10.1016/j.ensm.2018.05.009
Ding, F., Xu, W., Graff, G.L., et al.: Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013). https://doi.org/10.1021/ja312241y
Li, S.P., Fang, S., Dou, H., et al.: RbF as a dendrite-inhibiting additive in lithium metal batteries. ACS Appl. Mater. Interfaces 11, 20804–20811 (2019). https://doi.org/10.1021/acsami.9b03940
Goodman, J.K.S., Kohl, P.A.: Effect of alkali and alkaline earth metal salts on suppression of lithium dendrites. J. Electrochem. Soc. 161, D418–D424 (2014). https://doi.org/10.1149/2.0301409jes
Lu, Y.Y., Korf, K., Kambe, Y., et al.: Ionic-liquid-nanoparticle hybrid electrolytes: applications in lithium metal batteries. Angew. Chem. Int. Ed. 53, 488–492 (2014). https://doi.org/10.1002/anie.201307137
Rosso, M., Chazalviel, J.N., Chassaing, E.: Calculation of the space charge in electrodeposition from a binary electrolyte. J. Electroanal. Chem. 587, 323–328 (2006). https://doi.org/10.1016/j.jelechem.2005.11.030
Cai, Z.J., Liu, Y.B., Liu, S.S., et al.: High performance of lithium-ion polymer battery based on non-aqueous lithiated perfluorinated sulfonic ion-exchange membranes. Energy Environ. Sci. 5, 5690–5693 (2012). https://doi.org/10.1039/c1ee02708e
Srivastava, S., Schaefer, J.L., Yang, Z.C., et al.: Polymer-particle composites: phase stability and applications in electrochemical energy storage. Adv. Mater. 26, 201–234 (2014). https://doi.org/10.1002/adma.201303070
Lu, Y.Y., Das, S.K., Moganty, S.S., et al.: Ionic liquid-nanoparticle hybrid electrolytes and their application in secondary lithium-metal batteries. Adv. Mater. 24, 4430–4435 (2012). https://doi.org/10.1002/adma.201201953
Yan, K., Wang, J.Y., Zhao, S.Q., et al.: Temperature-dependent nucleation and growth of dendrite-free lithium metal anodes. Angew. Chem. Int. Ed. 58, 11364–11368 (2019). https://doi.org/10.1002/anie.201905251
Wang, J., Huang, W., Pei, A., et al.: Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 4, 664–670 (2019). https://doi.org/10.1038/s41560-019-0413-3
Li, L., Basu, S., Wang, Y.P., et al.: Self-heating-induced healing of lithium dendrites. Science 359, 1513–1516 (2018). https://doi.org/10.1126/science.aap8787
Mistry, A.N., Smith, K., Mukherjee, P.P.: Secondary-phase stochastics in lithium-ion battery electrodes. ACS Appl. Mater. Interfaces 10, 6317–6326 (2018). https://doi.org/10.1021/acsami.7b17771
Han, Y.H., Jie, Y.L., Huang, F.Y., et al.: Enabling stable lithium metal anode through electrochemical kinetics manipulation. Adv. Funct. Mater. 29, 1904629 (2019). https://doi.org/10.1002/adfm.201904629
Aryanfar, A., Brooks, D.J., Colussi, A.J., et al.: Thermal relaxation of lithium dendrites. Phys. Chem. Chem. Phys. 17, 8000–8005 (2015). https://doi.org/10.1039/c4cp05786d
Hong, Z.J., Viswanathan, V.: Prospect of thermal shock induced healing of lithium dendrite. ACS Energy Lett. 4, 1012–1019 (2019). https://doi.org/10.1021/acsenergylett.9b00433
Guo, Y.P., Li, D., Xiong, R.D., et al.: Investigation of the temperature-dependent behaviours of Li metal anode. Chem. Commun. 55, 9773–9776 (2019). https://doi.org/10.1039/c9cc04897a
Yang, H., Fey, E.O., Trimm, B.D., et al.: Effects of pulse plating on lithium electrodeposition, morphology and cycling efficiency. J. Power Sources 272, 900–908 (2014). https://doi.org/10.1016/j.jpowsour.2014.09.026
Aryanfar, A., Brooks, D., Merinov, B.V., et al.: Dynamics of lithium dendrite growth and inhibition: pulse charging experiments and Monte Carlo calculations. J. Phys. Chem. Lett. 5, 1721–1726 (2014). https://doi.org/10.1021/jz500207a
Li, Q., Tan, S., Li, L.L., et al.: Understanding the molecular mechanism of pulse current charging for stable lithium-metal batteries. Sci. Adv. 3, e1701246 (2017). https://doi.org/10.1126/sciadv.1701246
Shen, K., Wang, Z., Bi, X.X., et al.: Magnetic field–suppressed lithium dendrite growth for stable lithium-metal batteries. Adv. Energy Mater. 9, 1900260 (2019). https://doi.org/10.1002/aenm.201900260
Huang, A., Liu, H.D., Manor, O., et al.: Enabling rapid charging lithium metal batteries via surface acoustic wave-driven electrolyte flow. Adv. Mater. 32, 1907516 (2020). https://doi.org/10.1002/adma.201907516
Liu, X.M., Fang, A., Haataja, M.P., et al.: Size dependence of transport non-uniformities on localized plating in lithium-ion batteries. J. Electrochem. Soc. 165, A1147–A1155 (2018). https://doi.org/10.1149/2.1181805jes
Sheng, L., Wang, L., Wang, J.L., et al.: Accelerated lithium-ion conduction in covalent organic frameworks. Chem. Commun. 56, 10465–10468 (2020). https://doi.org/10.1039/d0cc04324a
Ryou, M.H., Lee, D.J., Lee, J.N., et al.: Excellent cycle life of lithium-metal anodes in lithium-ion batteries with mussel-inspired polydopamine-coated separators. Adv. Energy Mater. 2, 645–650 (2012). https://doi.org/10.1002/aenm.201100687
Li, Y.B., Sun, Y.M., Pei, A., et al.: Robust pinhole-free Li3N solid electrolyte grown from molten lithium. ACS Central Sci. 4, 97–104 (2018). https://doi.org/10.1021/acscentsci.7b00480
Han, B., Feng, D., Li, S., et al.: Self-regulated phenomenon of inorganic artificial solid electrolyte interphase for lithium metal batteries. Nano Lett. 20, 4029–4037 (2020). https://doi.org/10.1021/acs.nanolett.0c01400
Liang, J.W., Li, X.N., Zhao, Y., et al.: In situ Li3PS4 solid-state electrolyte protection layers for superior long-life and high-rate lithium-metal anodes. Adv. Mater. 30, 1804684 (2018). https://doi.org/10.1002/adma.201804684
Pang, Q., Liang, X., Shyamsunder, A., et al.: An in vivo formed solid electrolyte surface layer enables stable plating of Li metal. Joule 1, 871–886 (2017). https://doi.org/10.1016/j.joule.2017.11.009
Chen, H., Pei, A., Lin, D.C., et al.: Uniform high ionic conducting lithium sulfide protection layer for stable lithium metal anode. Adv. Energy Mater. 9, 1900858 (2019). https://doi.org/10.1002/aenm.201900858
Zhao, C.Z., Chen, P.Y., Zhang, R., et al.: An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 4, eaat3446 (2018). https://doi.org/10.1126/sciadv.aat3446
Song, J., Lee, H., Choo, M.J., et al.: Ionomer-liquid electrolyte hybrid ionic conductor for high cycling stability of lithium metal electrodes. Sci. Rep. 5, 14458 (2015). https://doi.org/10.1038/srep14458
Kim, M.S., Ryu, J.H., Deepika, et al.: Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 3, 889–898 (2018). https://doi.org/10.1038/s41560-018-0237-6
Luo, J., Fang, C.C., Wu, N.L.: Anodes: high polarity poly(vinylidene difluoride) thin coating for dendrite-free and high-performance lithium metal anodes. Adv. Energy Mater. 8, 1870008 (2018). https://doi.org/10.1002/aenm.201870008
Liang, X., Pang, Q., Kochetkov, I.R., et al.: A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2, 17119 (2017). https://doi.org/10.1038/nenergy.2017.119
Tu, Z., Choudhury, S., Zachman, M.J., et al.: Fast ion transport at solid–solid interfaces in hybrid battery anodes. Nat. Energy 3, 310–316 (2018). https://doi.org/10.1038/s41560-018-0096-1
Gunceler, D., Letchworth-Weaver, K., Sundararaman, R., et al.: The importance of nonlinear fluid response in joint density-functional theory studies of battery systems. Modelling Simul. Mater. Sci. Eng. 21, 074005 (2013). https://doi.org/10.1088/0965-0393/21/7/074005
Zhang, W.D., Wu, Q., Huang, J.X., et al.: Colossal granular lithium deposits enabled by the grain-coarsening effect for high-efficiency lithium metal full batteries. Adv. Mater. 32, 2001740 (2020). https://doi.org/10.1002/adma.202001740
Suo, L.M., Xue, W.J., Gobet, M., et al.: Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. PNAS 115, 1156–1161 (2018). https://doi.org/10.1073/pnas.1712895115
Gong, C., Pu, S.D., Gao, X.W., et al.: Revealing the role of fluoride-rich battery electrode interphases by operando transmission electron microscopy. Adv. Energy Mater. 11, 2003118 (2021). https://doi.org/10.1002/aenm.202003118
Chen, L., Chen, K.S., Chen, X.J., et al.: Novel ALD chemistry enabled low-temperature synthesis of lithium fluoride coatings for durable lithium anodes. ACS Appl. Mater. Interfaces 10, 26972–26981 (2018). https://doi.org/10.1021/acsami.8b04573
Li, N.W., Yin, Y.X., Yang, C.P., et al.: An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 28, 1853–1858 (2016). https://doi.org/10.1002/adma.201504526
Cheng, Q.H., He, W., Zhang, X.D., et al.: Recent advances in composite membranes modified with inorganic nanoparticles for high-performance lithium ion batteries. RSC Adv. 6, 10250–10265 (2016). https://doi.org/10.1039/c5ra21670b
Kozen, A.C., Lin, C.F., Pearse, A.J., et al.: Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 9, 5884–5892 (2015). https://doi.org/10.1021/acsnano.5b02166
Wang, L.P., Zhang, L., Wang, Q.J., et al.: Long lifespan lithium metal anodes enabled by Al2O3 sputter coating. Energy Storage Mater. 10, 16–23 (2018). https://doi.org/10.1016/j.ensm.2017.08.001
Lin, D.C., Liu, Y.Y., Chen, W., et al.: Conformal lithium fluoride protection layer on three-dimensional lithium by nonhazardous gaseous reagent Freon. Nano Lett. 17, 3731–3737 (2017). https://doi.org/10.1021/acs.nanolett.7b01020
Zhao, J., Liao, L., Shi, F.F., et al.: Surface fluorination of reactive battery anode materials for enhanced stability. J. Am. Chem. Soc. 139, 11550–11558 (2017). https://doi.org/10.1021/jacs.7b05251
Zhang, X.Q., Chen, X., Xu, R., et al.: Columnar lithium metal anodes. Angew. Chem. Int. Ed. 56, 14207–14211 (2017). https://doi.org/10.1002/anie.201707093
Fan, X., Chen, L., Borodin, O., et al.: Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnology 13, 715–722 (2018). https://doi.org/10.1038/s41565-018-0183-2
Zheng, G., Lee, S.W., Liang, Z., et al.: Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnology 9, 618–623 (2014). https://doi.org/10.1038/nnano.2014.152
Yang, Q.F., Cui, M.N., Hu, J.L., et al.: Ultrathin defective C-N coating to enable nanostructured Li plating for Li metal batteries. ACS Nano 14, 1866–1878 (2020). https://doi.org/10.1021/acsnano.9b08008
Subramanya, U., Chua, C., He Leong, V.G., et al.: Carbon-based artificial SEI layers for aqueous lithium-ion battery anodes. RSC Adv. 10, 674–681 (2020). https://doi.org/10.1039/c9ra08268a
Ye, S.F., Wang, L.F., Liu, F.F., et al.: G-C3N4 derivative artificial organic/inorganic composite solid electrolyte interphase layer for stable lithium metal anode. Adv. Energy Mater. 10, 2002647 (2020). https://doi.org/10.1002/aenm.202002647
He, M., Guo, R., Hobold, G.M., et al.: The intrinsic behavior of lithium fluoride in solid electrolyte interphases on lithium. P. Natl. Acad. Sci. 117, 73–79 (2020). https://doi.org/10.1073/pnas.1911017116
Liu, Y.Y., Lin, D.C., Yuen, P.Y., et al.: An artificial solid electrolyte interphase with high Li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Adv. Mater. 29, 1605531 (2017). https://doi.org/10.1002/adma.201605531
Jiang, S., Lu, Y., Lu, Y.Y., et al.: Nafion/titanium dioxide-coated lithium anode for stable lithium-sulfur batteries. Chem. Asian J. 13, 1379–1385 (2018). https://doi.org/10.1002/asia.201800326
Shen, C.F., Ge, M.Y., Zhang, A.Y., et al.: Silicon(lithiated)-sulfur full cells with porous silicon anode shielded by Nafion against polysulfides to achieve high capacity and energy density. Nano Energy 19, 68–77 (2016). https://doi.org/10.1016/j.nanoen.2015.11.013
Zhuang, T.Z., Huang, J.Q., Peng, H.J., et al.: Rational integration of polypropylene/graphene oxide/nafion as ternary-layered separator to retard the shuttle of polysulfides for lithium-sulfur batteries. Small 12, 381–389 (2016). https://doi.org/10.1002/smll.201503133
Luo, J., Lee, R.C., Jin, J.T., et al.: A dual-functional polymer coating on a lithium anode for suppressing dendrite growth and polysulfide shuttling in Li–S batteries. Chem. Commun. 53, 963–966 (2017). https://doi.org/10.1039/c6cc09248a
Qian, J., Henderson, W.A., Xu, W., et al.: High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015). https://doi.org/10.1038/ncomms7362
Piao, N., Ji, X., Xu, H., et al.: Countersolvent electrolytes for lithium-metal batteries. Adv. Energy Mater. 10, 1903568 (2020). https://doi.org/10.1002/aenm.201903568
Zheng, J.M., Lochala, J.A., Kwok, A., et al.: Research progress towards understanding the unique interfaces between concentrated electrolytes and electrodes for energy storage applications. Adv. Sci. 4, 1700032 (2017). https://doi.org/10.1002/advs.201700032
Yamada, Y., Yamada, A.: Review: superconcentrated electrolytes for lithium batteries. J. Electrochem. Soc. 162, A2406–A2423 (2015). https://doi.org/10.1149/2.0041514jes
Li, M., Wang, C.S., Chen, Z.W., et al.: New concepts in electrolytes. Chem. Rev. 120, 6783–6819 (2020). https://doi.org/10.1021/acs.chemrev.9b00531
Yamada, Y., Furukawa, K., Sodeyama, K., et al.: Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 136, 5039–5046 (2014). https://doi.org/10.1021/ja412807w
Fang, Z., Ma, Q., Liu, P., et al.: Novel concentrated Li[(FSO2)(n-C4F9SO2)N]-based ether electrolyte for superior stability of metallic lithium anode. ACS Appl. Mater. Interfaces 9, 4282–4289 (2017). https://doi.org/10.1021/acsami.6b03857
Fu, J.L., Ji, X., Chen, J., et al.: Lithium nitrate regulated sulfone electrolytes for lithium metal batteries. Angew. Chem. Int. Ed. 59, 22194–22201 (2020). https://doi.org/10.1002/anie.202009575
Chen, S.R., Zheng, J.M., Mei, D.H., et al.: High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, 1706102 (2018). https://doi.org/10.1002/adma.201706102
Ren, X.D., Chen, S.R., Lee, H., et al.: Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 4, 1877–1892 (2018). https://doi.org/10.1016/j.chempr.2018.05.002
Zeng, Z., Murugesan, V., Han, K.S., et al.: Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 3, 674–681 (2018). https://doi.org/10.1038/s41560-018-0196-y
Peng, Z., Cao, X., Gao, P.Y., et al.: High-power lithium metal batteries enabled by high-concentration acetonitrile-based electrolytes with vinylene carbonate additive. Adv. Funct. Mater. 30, 2001285 (2020). https://doi.org/10.1002/adfm.202001285
Yu, L., Chen, S.R., Lee, H., et al.: A localized high-concentration electrolyte with optimized solvents and lithium difluoro(oxalate)borate additive for stable lithium metal batteries. ACS Energy Lett. 3, 2059–2067 (2018). https://doi.org/10.1021/acsenergylett.8b00935
Ren, X.D., Zou, L.F., Cao, X., et al.: Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019). https://doi.org/10.1016/j.joule.2019.05.006
Wang, W., Zhang, J.L., Yang, Q., et al.: Stable cycling of high-voltage lithium-metal batteries enabled by high-concentration FEC-based electrolyte. ACS Appl. Mater. Interfaces 12, 22901–22909 (2020). https://doi.org/10.1021/acsami.0c03952
Chen, S.J., Xiang, Y.X., Zheng, G.R., et al.: High-efficiency lithium metal anode enabled by a concentrated/fluorinated ester electrolyte. ACS Appl. Mater. Interfaces 12, 27794–27802 (2020). https://doi.org/10.1021/acsami.0c06930
Fan, X.L., Chen, L., Ji, X., et al.: Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 4, 174–185 (2018). https://doi.org/10.1016/j.chempr.2017.10.017
Soto, F.A., Ma, Y.G., Martinez de la Hoz, J.M., et al.: Formation and growth mechanisms of solid-electrolyte interphase layers in rechargeable batteries. Chem. Mater. 27, 7990–8000 (2015). https://doi.org/10.1021/acs.chemmater.5b03358
Nilsson, V., Kotronia, A., Lacey, M., et al.: Highly concentrated LiTFSI–EC electrolytes for lithium metal batteries. ACS Appl. Energy Mater. 3, 200–207 (2020). https://doi.org/10.1021/acsaem.9b01203
Alvarado, J., Schroeder, M.A., Pollard, T.P., et al.: Bisalt ether electrolytes: a pathway towards lithium metal batteries with Ni-rich cathodes. Energy Environ. Sci. 12, 780–794 (2019). https://doi.org/10.1039/c8ee02601g
Liu, X.Y., Shen, C., Gao, N., et al.: Concentrated electrolytes based on dual salts of LiFSI and LiODFB for lithium-metal battery. Electrochim. Acta 289, 422–427 (2018). https://doi.org/10.1016/j.electacta.2018.09.085
Cheng, P.F., Zhang, H., Ma, Q., et al.: Highly salt-concentrated electrolyte comprising lithium bis(fluorosulfonyl)imide and 1,3-dioxolane-based ether solvents for 4-V-class rechargeable lithium metal cell. Electrochim. Acta 363, 137198 (2020). https://doi.org/10.1016/j.electacta.2020.137198
Hagos, T.T., Thirumalraj, B., Huang, C.J., et al.: Locally concentrated LiPF6 in a carbonate-based electrolyte with fluoroethylene carbonate as a diluent for anode-free lithium metal batteries. ACS Appl. Mater. Interfaces 11, 9955–9963 (2019). https://doi.org/10.1021/acsami.8b21052
Yuan, S.Y., Weng, S.T., Wang, F., et al.: Revisiting the designing criteria of advanced solid electrolyte interphase on lithium metal anode under practical condition. Nano Energy 83, 105847 (2021). https://doi.org/10.1016/j.nanoen.2021.105847
Fang, C., Lu, B., Pawar, G., et al.: Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat. Energy 6, 987–994 (2021). https://doi.org/10.1038/s41560-021-00917-3
Shin, W.K., Kim, D.W.: High performance ceramic-coated separators prepared with lithium ion-containing SiO2 particles for lithium-ion batteries. J. Power Sources 226, 54–60 (2013). https://doi.org/10.1016/j.jpowsour.2012.10.082
Jeong, H.S., Lee, S.Y.: Closely packed SiO2 nanoparticles/poly(vinylidene fluoride-hexafluoropropylene) layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 196, 6716–6722 (2011). https://doi.org/10.1016/j.jpowsour.2010.11.037
Miao, R.Y., Liu, B.W., Zhu, Z.Z., et al.: PVDF-HFP-based porous polymer electrolyte membranes for lithium-ion batteries. J. Power Sources 184, 420–426 (2008). https://doi.org/10.1016/j.jpowsour.2008.03.045
Hao, X.M., Zhu, J., Jiang, X., et al.: Ultrastrong polyoxyzole nanofiber membranes for dendrite-proof and heat-resistant battery separators. Nano Lett. 16, 2981–2987 (2016). https://doi.org/10.1021/acs.nanolett.5b05133
Zeng, X.X., Yin, Y.X., Li, N.W., et al.: Reshaping lithium plating/stripping behavior via bifunctional polymer electrolyte for room-temperature solid Li metal batteries. J. Am. Chem. Soc. 138, 15825–15828 (2016). https://doi.org/10.1021/jacs.6b10088
Monroe, C., Newman, J.: The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396 (2005). https://doi.org/10.1149/1.1850854
Tung, S.O., Ho, S., Yang, M., et al.: A dendrite-suppressing composite ion conductor from aramid nanofibres. Nat. Commun. 6, 6152 (2015). https://doi.org/10.1038/ncomms7152
Niu, C., Lee, H., Chen, S., et al.: High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 4, 551–559 (2019). https://doi.org/10.1038/s41560-019-0390-6
Wilkinson, D.P., Blom, H., Brandt, K., et al.: Effects of physical constraints on Li cyclability. J. Power Sources 36, 517–527 (1991). https://doi.org/10.1016/0378-7753(91)80077-B
Wilkinson, D.P., Wainwright, D.: In-situ study of electrode stack growth in rechargeable cells at constant pressure. J. Electroanal. Chem. 355, 193–203 (1993). https://doi.org/10.1016/0022-0728(93)80362-L
Ota, H., Shima, K., Ue, M., et al.: Effect of vinylene carbonate as additive to electrolyte for lithium metal anode. Electrochim. Acta 49, 565–572 (2004). https://doi.org/10.1016/j.electacta.2003.09.010
Lee, H., Chen, S.R., Ren, X.D., et al.: Electrode edge effects and the failure mechanism of lithium-metal batteries. Chemsuschem 11, 3821–3828 (2018). https://doi.org/10.1002/cssc.201801445
Wu, F., Quan, H., Han, J., et al.: Free-standing lithiophilic Ag-nanoparticle-decorated 3D porous carbon nanotube films for enhanced lithium storage. RSC Adv. 10, 30880–30886 (2020). https://doi.org/10.1039/d0ra04579a
Huang, Z.J., Zhou, G.M., Lv, W., et al.: Seeding lithium seeds towards uniform lithium deposition for stable lithium metal anodes. Nano Energy 61, 47–53 (2019). https://doi.org/10.1016/j.nanoen.2019.04.036
Lu, Y.Z., Wang, J.S., Chen, Y., et al.: Spatially controlled lithium deposition on silver-nanocrystals-decorated TiO2 nanotube arrays enabling ultrastable lithium metal anode. Adv. Funct. Mater. 31, 2009605 (2021). https://doi.org/10.1002/adfm.202009605
Yang, C.P., Yao, Y.G., He, S.M., et al.: Ultrafine silver nanoparticles for seeded lithium deposition toward stable lithium metal anode. Adv. Mater. 29, 1702714 (2017). https://doi.org/10.1002/adma.201702714
Ryou, M.H., Lee, Y.M., Lee, Y.J., et al.: Mechanical surface modification of lithium metal: towards improved Li metal anode performance by directed Li plating. Adv. Funct. Mater. 25, 834–841 (2015). https://doi.org/10.1002/adfm.201402953
Ma, Y.T., Wang, L.L., Fu, S.Y., et al.: In situ formation of a Li–Sn alloy protected layer for inducing lateral growth of dendrites. J. Mater. Chem. A 8, 23574–23579 (2020). https://doi.org/10.1039/d0ta08307k
Qu, J.L., Xiao, J.W., Wang, T.S., et al.: High rate transfer mechanism of lithium ions in lithium–tin and lithium–indium alloys for lithium batteries. J. Phys. Chem. C 124, 24644–24652 (2020). https://doi.org/10.1021/acs.jpcc.0c07880
Wan, M., Kang, S., Wang, L., et al.: Mechanical rolling formation of interpenetrated lithium metal/lithium tin alloy foil for ultrahigh-rate battery anode. Nat. Commun. 11, 829 (2020). https://doi.org/10.1038/s41467-020-14550-3
Liu, S.S., Ma, Y.L., Zhou, Z.X., et al.: Inducing uniform lithium nucleation by integrated lithium-rich Li-In anode with lithiophilic 3D framework. Energy Storage Mater. 33, 423–431 (2020). https://doi.org/10.1016/j.ensm.2020.08.007
Wan, J., Song, Y.X., Chen, W.P., et al.: Micromechanism in all-solid-state alloy-metal batteries: regulating homogeneous lithium precipitation and flexible solid electrolyte interphase evolution. J. Am. Chem. Soc. 143, 839–848 (2021). https://doi.org/10.1021/jacs.0c10121
Zhang, Y.Z., Sun, C.W.: Composite lithium protective layer formed in situ for stable lithium metal batteries. ACS Appl. Mater. Interfaces 13, 12099–12105 (2021). https://doi.org/10.1021/acsami.1c00745
Jia, J.Y., Tang, Z.Q., Guo, Z.X., et al.: A 3D composite lithium metal anode with pre-fabricated LiZn via reactive wetting. Chem. Commun. 56, 4248–4251 (2020). https://doi.org/10.1039/d0cc00514b
Chi, S.S., Wang, Q.R., Han, B., et al.: Lithiophilic Zn sites in porous CuZn alloy induced uniform Li nucleation and dendrite-free Li metal deposition. Nano Lett. 20, 2724–2732 (2020). https://doi.org/10.1021/acs.nanolett.0c00352
Zhong, H., Wu, Y.X., Ding, F., et al.: An artificial Li-Al interphase layer on Li-B alloy for stable lithium-metal anode. Electrochim. Acta 304, 255–262 (2019). https://doi.org/10.1016/j.electacta.2019.03.009
Li, H., Yamaguchi, T., Matsumoto, S., et al.: Circumventing huge volume strain in alloy anodes of lithium batteries. Nat. Commun. 11, 1584 (2020). https://doi.org/10.1038/s41467-020-15452-0
Zhuang, H.F., Zhao, P., Li, G.D., et al.: Li-LiAl alloy composite with memory effect as high-performance lithium metal anode. J. Power Sources 455, 227977 (2020). https://doi.org/10.1016/j.jpowsour.2020.227977
Tang, W., Yin, X.S., Kang, S.J., et al.: Lithium silicide surface enrichment: a solution to lithium metal battery. Adv. Mater. 30, 1801745 (2018). https://doi.org/10.1002/adma.201801745
Wang, H.S., Cao, X., Gu, H.K., et al.: Improving lithium metal composite anodes with seeding and pillaring effects of silicon nanoparticles. ACS Nano 14, 4601–4608 (2020). https://doi.org/10.1021/acsnano.0c00184
Yang, C.P., Xie, H., Ping, W.W., et al.: An electron/ion dual-conductive alloy framework for high-rate and high-capacity solid-state lithium-metal batteries. Adv. Mater. 31, 1804815 (2019). https://doi.org/10.1002/adma.201804815
Krauskopf, T., Mogwitz, B., Rosenbach, C., et al.: Diffusion limitation of lithium metal and Li-Mg alloy anodes on LLZO type solid electrolytes as a function of temperature and pressure. Adv. Energy Mater. 9, 1902568 (2019). https://doi.org/10.1002/aenm.201902568
Fu, K., Gong, Y.H., Fu, Z.Z., et al.: Transient behavior of the metal interface in lithium metal-garnet batteries. Angew. Chem. Int. Ed. 56, 14942–14947 (2017). https://doi.org/10.1002/anie.201708637
Lee, Y.G., Fujiki, S., Jung, C., et al.: High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy 5, 299–308 (2020). https://doi.org/10.1038/s41560-020-0575-z
Zhang, S.M., Yang, G.J., Liu, Z.P., et al.: Phase diagram determined lithium plating/stripping behaviors on lithiophilic substrates. ACS Energy Lett. 6, 4118–4126 (2021). https://doi.org/10.1021/acsenergylett.1c02127
Zhang, W.J.: Lithium insertion/extraction mechanism in alloy anodes for lithium-ion batteries. J. Power Sources 196, 877–885 (2011). https://doi.org/10.1016/j.jpowsour.2010.08.114
Acknowledgements
This work was funded by the Ministry of Science and Technology of China (No. 2019YFE0100200 and 2019YFA0705703), the National Natural Science Foundation of China (No. U1564205) and the Tsinghua University Initiative Scientific Research Program (No. 2019Z02UTY06 and 2019THFS0132). The authors also thank the Joint Work Plan for Research Projects under the Clean Vehicles Consortium at U.S. and the China-Clean Energy Research Center (CERC-CVC2.0, 2016–2020) and thank the Tsinghua University-Zhangjiagang Joint Institute for Hydrogen Energy and Lithium Ion Battery Technology.
Author information
Authors and Affiliations
Contributions
This work was funded by the Ministry of Science and Technology of China (No. 2019YFE0100200 and 2019YFA0705703), the National Natural Science Foundation of China (No. U1564205) and the Tsinghua University Initiative Scientific Research Program (No. 2019Z02UTY06 and 2019THFS0132). All authors ensure that the materials support the published claims and comply with field standards. All authors contributed to the study conception and design. Hongmei Liang, Li Wang and Xiangming He had the idea for the article. Li Sheng, Hong Xu and Youzhi Song performed the literature search. Hongmei Liang wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, H., Wang, L., Sheng, L. et al. Focus on the Electroplating Chemistry of Li Ions in Nonaqueous Liquid Electrolytes: Toward Stable Lithium Metal Batteries. Electrochem. Energy Rev. 5 (Suppl 2), 23 (2022). https://doi.org/10.1007/s41918-022-00158-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-022-00158-2