Abstract
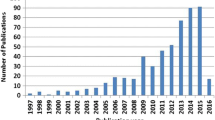
Dealloying has been recognized as a universal strategy to fabricate various functional electrode materials with open networks, nanoscale ligaments, tunable pore sizes and rich surface chemistry, all of which are very attractive characteristics for rechargeable lithium batteries. In particular, lithium ion insertion/extraction in metal anodes is naturally associated with the alloying/dealloying mechanism. The past decade has witnessed rapid growth of this research field with enormous progress. In this review article, we first summarize the recent development and microstructural regulation of dealloyed materials. Next, we focus on the rational design of nanoporous electrodes for rechargeable lithium batteries and related structure-performance correlations. Finally, some critical issues and perspectives are presented to guide the future development directions of such promising technology for high-energy batteries.
Graphic abstract
This review systematically summarizes the recent progress of dealloyed nanoporous materials and their application in rechargeable lithium batteries.













Similar content being viewed by others
References
Ding, Y., Kim, Y.J., Erlebacher, J.: Nanoporous gold leaf: “ancient technology”/advanced material. Adv. Mater. 16, 1897–1900 (2004). https://doi.org/10.1002/adma.200400792
Chen, Q., Ding, Y., Chen, M.: Nanoporous metal by dealloying for electrochemical energy conversion and storage. MRS Bull. 43, 43–48 (2018). https://doi.org/10.1557/mrs.2017.300
Li, R., Sieradzki, K.: Ductile-brittle transition in random porous Au. Phys. Rev. Lett. 68, 1168–1171 (1992). https://doi.org/10.1103/PhysRevLett.68.1168
Qian, L.H., Chen, M.W.: Ultrafine nanoporous gold by low-temperature dealloying and kinetics of nanopore formation. Appl. Phys. Lett. 91, 083105 (2007). https://doi.org/10.1063/1.2773757
Qi, Z., Weissmüller, J.: Hierarchical nested-network nanostructure by dealloying. ACS Nano 7, 5948–5954 (2013). https://doi.org/10.1021/nn4021345
Ding, Y., Erlebacher, J.: Nanoporous metals with controlled multimodal pore size distribution. J. Am. Chem. Soc. 125, 7772–7773 (2003). https://doi.org/10.1021/ja035318g
Lang, X., Hirata, A., Fujita, T., et al.: Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 6, 232–236 (2011). https://doi.org/10.1038/nnano.2011.13
Ding, Y., Chen, M.: Nanoporous metals for catalytic and optical applications. MRS Bull. 34, 569–576 (2011). https://doi.org/10.1557/mrs2009.156
Snyder, J., Asanithi, P., Dalton, A.B., et al.: Stabilized nanoporous metals by dealloying ternary alloy precursors. Adv. Mater. 20, 4883–4886 (2008). https://doi.org/10.1002/adma.200702760
Zhang, J., Li, C.M.: Nanoporous metals: fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev. 41, 7016–7031 (2012). https://doi.org/10.1039/C2CS35210A
Guo, D.J., Ding, Y.: Porous nanostructured metals for electrocatalysis. Electroanalysis 24, 2035–2043 (2012). https://doi.org/10.1002/elan.201200450
Jin, H.J., Wang, X.L., Parida, S., et al.: Nanoporous Au–Pt alloys as large strain electrochemical actuators. Nano Lett. 10, 187–194 (2010). https://doi.org/10.1021/nl903262b
Detsi, E., Sellès, M.S., Onck, P.R., et al.: Nanoporous silver as electrochemical actuator. Scripta Mater. 69, 195–198 (2013). https://doi.org/10.1016/j.scriptamat.2013.04.003
Biener, J., Wittstock, A., Zepeda-Ruiz, L.A., et al.: Surface-chemistry-driven actuation in nanoporous gold. Nat. Mater. 8, 47–51 (2009). https://doi.org/10.1038/nmat2335
Wittstock, A., Biener, J., Bäumer, M.: Nanoporous gold: a new material for catalytic and sensor applications. Phys. Chem. Chem. Phys. 12, 12919–12930 (2010). https://doi.org/10.1039/C0CP00757A
Zhang, L., Chang, H., Hirata, A., et al.: Nanoporous gold based optical sensor for sub-ppt detection of mercury ions. ACS Nano 7, 4595–4600 (2013). https://doi.org/10.1021/nn4013737
Wang, J., Gao, H., Sun, F., et al.: Nanoporous PtAu alloy as an electrochemical sensor for glucose and hydrogen peroxide. Sensors Actuators B Chem. 191, 612–618 (2014). https://doi.org/10.1016/j.snb.2013.10.034
Oh, J., Deutsch, T.G., Yuan, H.-C., et al.: Nanoporous black silicon photocathode for H2 production by photoelectrochemical water splitting. Energy Environ. Sci. 4, 1690–1694 (2011). https://doi.org/10.1039/C1EE01124C
Bak, C.H., Kim, K., Jung, K., et al.: Efficient photoelectrochemical water splitting of nanostructured hematite on a three-dimensional nanoporous metal electrode. J. Mater. Chem. A 2, 17249–17252 (2014). https://doi.org/10.1039/C4TA03578J
Yang, Y., Ruan, G., Xiang, C., et al.: Flexible three-dimensional nanoporous metal-based energy devices. J. Am. Chem. Soc. 136, 6187–6190 (2014). https://doi.org/10.1021/ja501247f
Polat, O., Seker, E.: Halide-gated molecular release from nanoporous gold thin films. J. Phys. Chem. C 119, 24812–24818 (2015). https://doi.org/10.1021/acs.jpcc.5b06959
Garcia-Gradilla, V., Sattayasamitsathit, S., Soto, F., et al.: Ultrasound-propelled nanoporous gold wire for efficient drug loading and release. Small 10, 4154–4159 (2014). https://doi.org/10.1002/smll.201401013
Seker, E., Berdichevsky, Y., Staley, K.J., et al.: Microfabrication-compatible nanoporous gold foams as biomaterials for drug delivery. Adv. Healthc. Mater. 1, 172–176 (2012). https://doi.org/10.1002/adhm.201200002
Zhang, Z., Wang, Y., Qi, Z., et al.: Generalized fabrication of nanoporous metals (Au, Pd, Pt, Ag, and Cu) through chemical dealloying. J. Phys. Chem. C 113, 12629–12636 (2009). https://doi.org/10.1021/jp811445a
Rebbecchi, T.A., Chen, Y.: Template-based fabrication of nanoporous metals. J. Mater. Res. 33, 2–15 (2017). https://doi.org/10.1557/jmr.2017.383
Nishio, K., Masuda, H.: Anodization of gold in oxalate solution to form a nanoporous black film. Angew. Chem. Int. Ed. 50, 1603–1607 (2011). https://doi.org/10.1002/anie.201005700
Näth, O., Stephen, A., Rösler, J., et al.: Structuring of nanoporous nickel-based superalloy membranes via laser etching. J. Mater. Process. Technol. 209, 4739–4743 (2009). https://doi.org/10.1016/j.jmatprotec.2008.11.042
Kazanskiy, N.L., Murzin, S.P., Osetrov, Y.L., et al.: Synthesis of nanoporous structures in metallic materials under laser action. Opt. Laser. Eng. 49, 1264–1267 (2011). https://doi.org/10.1016/j.optlaseng.2011.07.001
Tappan, B.C., Steiner III, S.A., Luther, E.P.: Nanoporous metal foams. Angew. Chem. Int. Ed. 49, 4544–4565 (2010). https://doi.org/10.1002/anie.200902994
Tappan, B.C., Huynh, M.H., Hiskey, M.A., et al.: Ultralow-density nanostructured metal foams: combustion synthesis, morphology, and composition. J. Am. Chem. Soc. 128, 6589–6594 (2006). https://doi.org/10.1021/ja056550k
Avisar-Levy, M., Levy, O., Ascarelli, O., et al.: Fractal structures of highly-porous metals and alloys at the nanoscale. J. Alloys Compd. 635, 48–54 (2015). https://doi.org/10.1016/j.jallcom.2015.02.073
Zhang, X., Guan, P., Malic, L., et al.: Nanoporous twinned PtPd with highly catalytic activity and stability. J. Mater. Chem. A 3, 2050–2056 (2015). https://doi.org/10.1039/C4TA06250G
Ding, Y., Zhang, Z.: Formation and microstructural regulation of nanoporous metals. In: Nanoporous Metals for Advanced Energy Technologies, pp. 37–81. Springer, Cham (2016)
Huber, G.W., Shabaker, J.W., Dumesic, J.A.: Raney Ni-Sn catalyst for H2production from biomass-derived hydrocarbons. Science 300, 2075–2077 (2003). https://doi.org/10.1126/science.1085597
Raney nickel catalyst market report 2019-global and Chinese market size, share & trends analysis, by manufacturers, products, applications. IOP Publishing PhysicsWeb. https://www.prof-research.com/index.phproute=product/product&product_id=81238(2020). Accessed 13 Jan 2020
Weissmüller, J., Sieradzki, K.: Dealloyed nanoporous materials with interface-controlled behavior. MRS Bull. 43, 14–19 (2018). https://doi.org/10.1557/mrs.2017.299
Kunduraci, M.: Dealloying technique in the synthesis of lithium-ion battery anode materials. J. Solid State Electrochem. 20, 2105–2111 (2016). https://doi.org/10.1007/s10008-016-3226-3
Song, T., Yan, M., Qian, M.: The enabling role of dealloying in the creation of specific hierarchical porous metal structures—a review. Corros. Sci. 134, 78–98 (2018). https://doi.org/10.1016/j.corsci.2018.02.013
Hu, Y.-S., Guo, Y.G., Sigle, W., et al.: Electrochemical lithiation synthesis of nanoporous materials with superior catalytic and capacitive activity. Nat. Mater. 5, 713–717 (2006). https://doi.org/10.1038/nmat1709
Liu, X.H., Huang, S., Picraux, S.T., et al.: Reversible Nanopore formation in Ge nanowires during lithiation–delithiation cycling: an in Situ transmission electron microscopy study. Nano Lett. 11, 3991–3997 (2011). https://doi.org/10.1021/nl2024118
Chen, Q., Sieradzki, K.: Spontaneous evolution of bicontinuous nanostructures in dealloyed Li-based systems. Nat. Mater. 12, 1102–1106 (2013). https://doi.org/10.1038/nmat3741
Tavassol, H., Cason, M.W., Nuzzo, R.G., et al.: Influence of oxides on the stress evolution and reversibility during SnOx conversion and Li-Sn alloying reactions. Adv. Energy Mater. 5, 1400317 (2015). https://doi.org/10.1002/aenm.201400317
Xia, H., Lai, M.O., Lu, L.: Nanoporous MnOx thin-film electrodes synthesized by electrochemical lithiation/delithiation for supercapacitors. J. Power Sources 196, 2398–2402 (2011). https://doi.org/10.1016/j.jpowsour.2010.09.032
Nishio, K., Yoshida, M., Masuda, H.: Fabrication of nanoporous Pt by electrochemical alloying and dealloying with Li. ECS Electrochem. Lett. 2, C43–C45 (2013). https://doi.org/10.1149/2.007311eel
Hu, Y., Zhang, T., Cheng, F.: Recycling application of Li–MnO2 batteries as rechargeable lithium-air batteries. Angew. Chem. Int. Ed. 54, 4338–4343 (2015). https://doi.org/10.1002/anie.201411626
Kennedy, T., Mullane, E., Geaney, H., et al.: High-performance Germanium nanowire-based lithium-ion battery anodes extending over 1000 cycles through in Situ formation of a continuous porous network. Nano Lett. 14, 716–723 (2014). https://doi.org/10.1021/nl403979s
Lian, Q., Zhou, G., Liu, J., et al.: Extrinsic pseudocapacitve Li-ion storage of SnS anode via lithiation-induced structural optimization on cycling. J. Power Sources 366, 1–8 (2017). https://doi.org/10.1016/j.jpowsour.2017.09.009
Cao, B., Liu, Z., Xu, C., et al.: High-rate-induced capacity evolution of mesoporous C@SnO2@C hollow nanospheres for ultra-long cycle lithium-ion batteries. J. Power Sources 414, 233–241 (2019). https://doi.org/10.1016/j.jpowsour.2019.01.001
Juarez, T., Biener, J., Weissmüller, J., et al.: Nanoporous metals with structural hierarchy: areview. Adv. Energy. Mater. 19, 1700389 (2017). https://doi.org/10.1002/adem.201700389
Hakamada, M., Mabuchi, M.: Fabrication, microstructure, and properties of nanoporous Pd, Ni, and their alloys by dealloying. Crit. Rev. Solid State Mater. Sci. 38, 262–285 (2013). https://doi.org/10.1080/10408436.2012.674985
McCue, I., Benn, E., Gaskey, B., et al.: Dealloying and dealloyed materials. Annu. Rev. Mater. Res. 46, 263–286 (2016). https://doi.org/10.1146/annurev-matsci-070115-031739
Meng, F., Ding, Y.: Sub-micrometer-thick all-solid-state supercapacitors with high power and energy densities. Adv. Mater. 23, 4098–4102 (2011). https://doi.org/10.1002/adma.201101678
Qian, L., Ding, Y., Fujita, T., et al.: Synthesis and optical properties of three-dimensional porous core-shell nanoarchitectures. Langmuir 24, 4426–4429 (2008). https://doi.org/10.1021/la703621c
Ding, Y., Zhang, Z.: Nanoporous metals for supercapacitor applications. In: Nanoporous Metals for Advanced Energy Technologies, pp. 137–173. Springer, Cham (2016)
Liu, L., Lyu, J., Zhao, T., et al.: Preparations and properties of porous copper materials for lithium-ion battery applications. Chem. Eng. Commun. 203, 707–713 (2016). https://doi.org/10.1080/00986445.2015.1104504
Erlebacher, J., Aziz, M.J., Karma, A., et al.: Evolution of nanoporosity in dealloying. Nature 410, 450–453 (2001). https://doi.org/10.1038/35068529
Weissmüller, J., Newman, R.C., Jin, H.J., et al.: Nanoporous metals by alloy corrosion: formation and mechanical properties. MRS Bull. 34, 577–586 (2011). https://doi.org/10.1557/mrs2009.157
McCue, I., Benn, E., Gaskey, B., et al.: Dealloying and dealloyed materials. Ann Rev Mater Res 46, 263–286 (2016). https://doi.org/10.1146/annurev-matsci-070115-031739
Xu, C., Su, J., Xu, X., et al.: Low temperature CO oxidation over unsupported nanoporous gold. J. Am. Chem. Soc. 129, 42–43 (2007). https://doi.org/10.1021/ja0675503
Fujita, T., Qian, L.H., Inoke, K., et al.: Three-dimensional morphology of nanoporous gold. Appl. Phys. Lett. 92, 251902 (2008). https://doi.org/10.1063/1.2948902
Wada, T., Yubuta, K., Inoue, A., et al.: Dealloying by metallic melt. Mater. Lett. 65, 1076–1078 (2011). https://doi.org/10.1016/j.matlet.2011.01.054
Gaskey, B., McCue, I., Chuang, A., et al.: Self-assembled porous metal-intermetallic nanocomposites via liquid metal dealloying. Acta Mater. 164, 293–300 (2019). https://doi.org/10.1016/j.actamat.2018.10.057
Wada, T., Yamada, J., Kato, H.: Preparation of three-dimensional nanoporous Si using dealloying by metallic melt and application as a lithium-ion rechargeable battery negative electrode. J. Power Sources 306, 8–16 (2016). https://doi.org/10.1016/j.jpowsour.2015.11.079
Sun, Y., Ren, Y.: New preparation method of porous copper powder through vacuum dealloying. Vacuum 122, 215–217 (2015). https://doi.org/10.1016/j.vacuum.2015.09.031
Sun, Y., Ren, Y., Yang, K.: New preparation method of micron porous copper through physical vacuum dealloying of Cu–Zn alloys. Mater. Lett. 165, 1–4 (2016). https://doi.org/10.1016/j.matlet.2015.11.102
Parida, S., Kramer, D., Volkert, C.A., et al.: Volume change during the formation of nanoporous gold by dealloying. Phys. Rev. Lett. 97, 035504 (2006). https://doi.org/10.1103/PhysRevLett.97.035504
Yu, J., Ding, Y., Xu, C., et al.: Nanoporous metals by dealloying multicomponent metallic glasses. Chem. Mater. 20, 4548–4550 (2008). https://doi.org/10.1021/cm8009644
Renner, F.U., Stierle, A., Dosch, H., et al.: Initial corrosion observed on the atomic scale. Nature 439, 707–710 (2006). https://doi.org/10.1038/nature04465
Yeh, F.H., Tai, C.C., Huang, J.F., et al.: Formation of porous silver by electrochemical alloying/dealloying in a water-insensitive zinc chloride-1-ethyl-3-methyl imidazolium chloride ionic liquid. J. Phys. Chem. B 110, 5215–5222 (2006). https://doi.org/10.1021/jp0552527
Xu, J., Zhang, C., Wang, X., et al.: Fabrication of bi-modal nanoporous bimetallic Pt–Au alloy with excellent electrocatalytic performance towards formic acid oxidation. Green Chem. 13, 1914–1922 (2011). https://doi.org/10.1039/C1GC15208D
Gan, L., Heggen, M., O’Malley, R., et al.: Understanding and controlling nanoporosity formation for improving the stability of bimetallic fuel cell catalysts. Nano Lett. 13, 1131–1138 (2013). https://doi.org/10.1021/nl304488q
Zhang, H., Wang, Z., Yang, M., et al.: The effect of an external magnetic field on the dealloying process of the Ni–Al alloy in alkaline solution. Phys. Chem. Chem. Phys. 19, 18167–18171 (2017). https://doi.org/10.1039/C7CP03363J
Xu, H., Shen, K., Liu, S., et al.: Micromorphology and phase composition manipulation of nanoporous gold with high methanol electro-oxidation catalytic activity through adding a magnetic field in the dealloying process. J. Phys. Chem. C 122, 3371–3385 (2018). https://doi.org/10.1021/acs.jpcc.7b10475
Cheng, I.C., Hodge, A.M.: High temperature morphology and stability of nanoporous Ag foams. J. Porous Mater. 21, 467–474 (2014). https://doi.org/10.1007/s10934-014-9793-8
Kertis, F., Snyder, J., Govada, L., et al.: Structure/processing relationships in the fabrication of nanoporous gold. JOM 62, 50–56 (2010). https://doi.org/10.1007/s11837-010-0087-6
Chen-Wiegart, Y.C.K., Wang, S., Chu, Y.S., et al.: Structural evolution of nanoporous gold during thermal coarsening. Acta Mater. 60, 4972–4981 (2012). https://doi.org/10.1016/j.actamat.2012.05.012
Shui, J.L., Zhang, J.W., Li, J.C.M.: Making Pt-shell Pt30Ni70 nanowires by mild dealloying and heat treatments with little Ni loss. J. Mater. Chem. 21, 6225–6229 (2011). https://doi.org/10.1039/C1JM10216H
Vega, A.A., Newman, R.C.: Beneficial effects of adsorbate-induced surface segregation of Pt in nanoporous metals fabricated by dealloying of Ag–Au–Pt alloys. J. Electrochem. Soc. 161, C11–C19 (2014). https://doi.org/10.1149/2.014401jes
Liu, D., Yang, Z., Wang, P., et al.: Preparation of 3D nanoporous copper-supported cuprous oxide for high-performance lithium ion battery anodes. Nanoscale 5, 1917–1921 (2013). https://doi.org/10.1039/C2NR33383J
Xu, Z.L., Liu, X., Luo, Y., et al.: Nanosilicon anodes for high performance rechargeable batteries. Prog. Mater Sci. 90, 1–44 (2017). https://doi.org/10.1016/j.pmatsci.2017.07.003
Ellis, B.L., Nazar, L.F.: Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid State Mater. Sci. 16, 168–177 (2012). https://doi.org/10.1016/j.cossms.2012.04.002
Wu, X., Xing, Z., Zhao, Y., et al.: Insert Zn nanoparticles into the 3D porous carbon ultrathin films as a superior anode material for lithium ion battery. Part. Part. Syst. Char. 35, 1700355 (2018). https://doi.org/10.1002/ppsc.201700355
Wu, X., Zhao, W., Wang, H., et al.: Enhanced capacity of chemically bonded phosphorus/carbon composite as an anode material for potassium-ion batteries. J. Power Sources 378, 460–467 (2018). https://doi.org/10.1016/j.jpowsour.2017.12.077
Ito, Y., Qiu, H.-J., Fujita, T., et al.: Bicontinuous nanoporous N-doped graphene for the oxygen reduction reaction. Adv. Mater. 26, 4145–4150 (2014). https://doi.org/10.1002/adma.201400570
Ito, Y., Tanabe, Y., Qiu, H.J., et al.: High-quality three-dimensional nanoporous graphene. Angew. Chem. Int. Ed. 53, 4822–4826 (2014). https://doi.org/10.1002/anie.201402662
Fan, W., Liu, X., Wang, Z., et al.: Synergetic enhancement of the electronic/ionic conductivity of a Li-ion battery by fabrication of a carbon-coated nanoporous SnOxSb alloy anode. Nanoscale 10, 7605–7611 (2018). https://doi.org/10.1039/C8NR00550H
Chen, Z., Ye, J., Qin, R., et al.: Carbon particles modified macroporous Si/Ni composite as an advanced anode material for lithium ion batteries. Int. J. Hydrogen Energy 44, 1078–1087 (2019). https://doi.org/10.1016/j.ijhydene.2018.11.065
Feng, J., Zhang, Z., Ci, L., et al.: Chemical dealloying synthesis of porous silicon anchored by in situ generated graphene sheets as anode material for lithium-ion batteries. J. Power Sources 287, 177–183 (2015). https://doi.org/10.1016/j.jpowsour.2015.04.051
Huang, K., Xing, Z., Wang, L., et al.: Direct synthesis of 3D hierarchically porous carbon/Sn composites via in situ generated NaCl crystals as templates for potassium-ion batteries anode. J. Mater. Chem. A 6, 434–442 (2018). https://doi.org/10.1039/C7TA08171E
Yu, Y., Gu, L., Lang, X., et al.: Li storage in 3D nanoporous Au-supported nanocrystalline tin. Adv. Mater. 23, 2443–2447 (2011). https://doi.org/10.1002/adma.201004331
Zhang, S., Xing, Y., Jiang, T., et al.: A three-dimensional tin-coated nanoporous copper for lithium-ion battery anodes. J. Power Sources 196, 6915–6919 (2011). https://doi.org/10.1016/j.jpowsour.2010.12.021
Hao, Q., Hou, J., Ye, J., et al.: Hierarchical macroporous Si/Sn composite: easy preparation and optimized performances towards lithium storage. Electrochim. Acta 306, 427–436 (2019). https://doi.org/10.1016/j.electacta.2019.03.163
Guo, X., Han, J., Liu, P., et al.: Hierarchical nanoporosity enhanced reversible capacity of bicontinuous nanoporous metal based Li–O2 battery. Sci. Rep. 6, 33466 (2016). https://doi.org/10.1038/srep33466
Lu, L., Han, X., Li, J., et al.: A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 226, 272–288 (2013). https://doi.org/10.1016/j.jpowsour.2012.10.060
Etacheri, V., Marom, R., Elazari, R., et al.: Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 4, 3243–3262 (2011). https://doi.org/10.1039/C1EE01598B
Poizot, P., Laruelle, S., Grugeon, S., et al.: Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000). https://doi.org/10.1038/35035045
Zhang, W.J.: A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 196, 13–24 (2011). https://doi.org/10.1016/j.jpowsour.2010.07.020
Obrovac, M.N., Chevrier, V.L.: Alloy negative electrodes for Li-ion batteries. Chem. Rev. 114, 11444–11502 (2014). https://doi.org/10.1021/cr500207g
Kasavajjula, U., Wang, C., Appleby, A.J.: Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 163, 1003–1039 (2007). https://doi.org/10.1016/j.jpowsour.2006.09.084
Szczech, J.R., Jin, S.: Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 4, 56–72 (2011). https://doi.org/10.1039/C0EE00281J
Chae, S., Choi, S.-H., Kim, N., et al.: Integration of graphite and silicon anodes for the commercialization of high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 59, 110–135 (2020). https://doi.org/10.1002/anie.201902085
Sharma, R.A., Seefurth, R.N.: Thermodynamic properties of the lithium-silicon system. J. Electrochem. Soc. 123, 1763–1768 (1976). https://doi.org/10.1149/1.2132692
Soto, F.A., Ma, Y., Martinez de la Hoz, J.M., et al.: Formation and growth mechanisms of solid-electrolyte interphase layers in rechargeable batteries. Chem. Mater. 27, 7990–8000 (2015). https://doi.org/10.1021/acs.chemmater.5b03358
Su, X., Wu, Q., Li, J., et al.: Silicon-based nanomaterials for lithium-ion batteries: a review. Adv. Energy Mater. 4, 1300882 (2014). https://doi.org/10.1002/aenm.201300882
He, W., Tian, H., Xin, F., et al.: Scalable fabrication of micro-sized bulk porous Si from Fe–Si alloy as a high performance anode for lithium-ion batteries. J. Mater. Chem. A 3, 17956–17962 (2015). https://doi.org/10.1039/C5TA04857E
Liang, J., Li, X., Hou, Z., et al.: Nanoporous silicon prepared through air-oxidation demagnesiation of Mg2Si and properties of its lithium ion batteries. Chem. Commun. 51, 7230–7233 (2015). https://doi.org/10.1039/C5CC01659B
Sohn, M., Lee, D.G., Park, H.I., et al.: Microstructure controlled porous silicon particles as a high capacity lithium storage material via dual step pore engineering. Adv. Funct. Mater. 28, 1800855 (2018). https://doi.org/10.1002/adfm.201800855
Terranova, M.L., Orlanducci, S., Tamburri, E., et al.: Si/C hybrid nanostructures for Li-ion anodes: an overview. J. Power Sources 246, 167–177 (2014). https://doi.org/10.1016/j.jpowsour.2013.07.065
Rahman, M.A., Song, G., Bhatt, A.I., et al.: Nanostructured silicon anodes for high-performance lithium-ion batteries. Adv. Funct. Mater. 26, 647–678 (2016). https://doi.org/10.1002/adfm.201502959
Zhang, L., Liu, X., Zhao, Q., et al.: Si-containing precursors for Si-based anode materials of Li-ion batteries: a review. Energy Storage Mater. 4, 92–102 (2016). https://doi.org/10.1016/j.ensm.2016.01.011
Yin, Y., Wan, L., Guo, Y.: Silicon-based nanomaterials for lithium-ion batteries. Chin. Sci. Bull. 57, 4104–4110 (2012). https://doi.org/10.1007/s11434-012-5017-2
Zuo, X., Zhu, J., Müller-Buschbaum, P., et al.: Silicon based lithium-ion battery anodes: a chronicle perspective review. Nano Energy 31, 113–143 (2017). https://doi.org/10.1016/j.nanoen.2016.11.013
An, Y., Fei, H., Zeng, G., et al.: Green, scalable, and controllable fabrication of nanoporous silicon from commercial alloy precursors for high-energy lithium-ion batteries. ACS Nano 12, 4993–5002 (2018). https://doi.org/10.1021/acsnano.8b02219
Wada, T., Ichitsubo, T., Yubuta, K., et al.: Bulk-nanoporous-silicon negative electrode with extremely high cyclability for lithium-ion batteries prepared using a top-down process. Nano Lett. 14, 4505–4510 (2014). https://doi.org/10.1021/nl501500g
Saager, S., Scheffel, B., Zywitzki, O., et al.: Porous silicon thin films as anodes for lithium ion batteries deposited by co-evaporation of silicon and zinc. Surf. Coat. Technol. 358, 586–593 (2019). https://doi.org/10.1016/j.surfcoat.2018.11.064
Zhao, C., Wada, T., De Andrade, V., et al.: Imaging of 3D morphological evolution of nanoporous silicon anode in lithium ion battery by X-ray nano-tomography. Nano Energy 52, 381–390 (2018). https://doi.org/10.1016/j.nanoen.2018.08.009
Jiang, Z., Li, C., Hao, S., et al.: An easy way for preparing high performance porous silicon powder by acid etching Al–Si alloy powder for lithium ion battery. Electrochim. Acta 115, 393–398 (2014). https://doi.org/10.1016/j.electacta.2013.08.123
Profatilova, I.A., Stock, C., Schmitz, A., et al.: Enhanced thermal stability of a lithiated nano-silicon electrode by fluoroethylene carbonate and vinylene carbonate. J. Power Sources 222, 140–149 (2013). https://doi.org/10.1016/j.jpowsour.2012.08.066
Jiang, T., Zhang, R., Yin, Q., et al.: Morphology, composition and electrochemistry of a nano-porous silicon versus bulk silicon anode for lithium-ion batteries. J. Mater. Sci. 52, 3670–3677 (2017). https://doi.org/10.1007/s10853-016-0599-8
Hwang, G., Kim, J.M., Hong, D., et al.: Multifunctional natural agarose as an alternative material for high-performance rechargeable lithium-ion batteries. Green Chem. 18, 2710–2716 (2016). https://doi.org/10.1039/C5GC02654G
Wu, X., Chen, Y., Xing, Z., et al.: Advanced carbon-based anodes for potassium-ion batteries. Adv. Energy Mater. 9, 1900343 (2019). https://doi.org/10.1002/aenm.201900343
Lv, X., Wei, W., Huang, B., et al.: Achieving high energy density for lithium-ion battery anodes by Si/C nanostructure design. J. Mater. Chem. A 7, 2165–2171 (2019). https://doi.org/10.1039/C8TA10936B
Park, H.I., Sohn, M., Kim, D.S., et al.: Carbon nanofiber/3D nanoporous silicon hybrids as high capacity lithium storage materials. ChemSusChem 9, 834–840 (2016). https://doi.org/10.1002/cssc.201501633
Jia, H., Zheng, J., Song, J., et al.: A novel approach to synthesize micrometer-sized porous silicon as a high performance anode for lithium-ion batteries. Nano Energy 50, 589–597 (2018). https://doi.org/10.1016/j.nanoen.2018.05.048
Zhou, W., Jiang, T., Zhou, H., et al.: The nanostructure of the Si–Al eutectic and its use in lithium batteries. MRS Commun. 3, 119–121 (2013). https://doi.org/10.1557/mrc.2013.20
Nguyen, H.T., Zamfir, M.R., Duong, L.D., et al.: Alumina-coated silicon-based nanowire arrays for high quality Li-ion battery anodes. J. Mater. Chem. 22, 24618–24626 (2012). https://doi.org/10.1039/C2JM35125K
He, Y., Yu, X., Wang, Y., et al.: Alumina-coated patterned amorphous silicon as the anode for a lithium-ion battery with high coulombic efficiency. Adv. Mater. 23, 4938–4941 (2011). https://doi.org/10.1002/adma.201102568
Hwang, G., Park, H., Bok, T., et al.: A high-performance nanoporous Si/Al2O3 foam lithium-ion battery anode fabricated by selective chemical etching of the Al–Si alloy and subsequent thermal oxidation. Chem. Commun. 51, 4429–4432 (2015). https://doi.org/10.1039/C4CC09956G
Hao, Q., Zhao, D., Duan, H., et al.: Si/Ag composite with bimodal micro-nano porous structure as a high-performance anode for Li-ion batteries. Nanoscale 7, 5320–5327 (2015). https://doi.org/10.1039/C4NR07384C
Xu, C., Hao, Q., Zhao, D.: Facile fabrication of a nanoporous Si/Cu composite and its application as a high-performance anode in lithium-ion batteries. Nano Res. 9, 908–916 (2016). https://doi.org/10.1007/s12274-015-0973-x
Ma, W., Liu, X., Wang, X., et al.: Crystalline Cu-silicide stabilizes the performance of a high capacity Si-based Li-ion battery anode. J. Mater. Chem. A 4, 19140–19146 (2016). https://doi.org/10.1039/C6TA08740J
Wang, J., Du, N., Zhang, H., et al.: Cu-Si1−xGex core-shell nanowire arrays as three-dimensional electrodes for high-rate capability lithium-ion batteries. J. Power Sources 208, 434–439 (2012). https://doi.org/10.1016/j.jpowsour.2012.02.039
Kim, D., Li, N., Sheehan, C.J., et al.: Degradation of Si/Ge core/shell nanowire heterostructures during lithiation and delithiation at 0.8 and 20 A g−1. Nanoscale 10, 7343–7351 (2018). https://doi.org/10.1039/C8NR00865E
Lin, N., Wang, L., Zhou, J., et al.: A Si/Ge nanocomposite prepared by a one-step solid-state metathesis reaction and its enhanced electrochemical performance. J. Mater. Chem. A 3, 11199–11202 (2015). https://doi.org/10.1039/C5TA02216A
Luo, W., Shen, D., Zhang, R., et al.: Germanium nanograin decoration on carbon shell: boosting lithium-storage properties of silicon nanoparticles. Adv. Funct. Mater. 26, 7800–7806 (2016). https://doi.org/10.1002/adfm.201603335
Wu, S., Han, C., Iocozzia, J., et al.: Germanium-based nanomaterials for rechargeable batteries. Angew. Chem. Int. Ed. 55, 7898–7922 (2016). https://doi.org/10.1002/anie.201509651
Yang, Y., Liu, S., Bian, X., et al.: Morphology- and porosity-tunable synthesis of 3D nanoporous SiGe alloy as a high-performance lithium-ion battery anode. ACS Nano 12, 2900–2908 (2018). https://doi.org/10.1021/acsnano.8b00426
Ye, J., Chen, Z., Hao, Q., et al.: One-step mild fabrication of porous core-shelled Si@TiO2 nanocomposite as high performance anode for Li-ion batteries. J. Colloid Interface Sci. 536, 171–179 (2019). https://doi.org/10.1016/j.jcis.2018.10.029
Tian, H., Tan, X., Xin, F., et al.: Micro-sized nano-porous Si/C anodes for lithium ion batteries. Nano Energy 11, 490–499 (2015). https://doi.org/10.1016/j.nanoen.2014.11.031
Wang, J., Meng, X., Fan, X., et al.: Scalable synthesis of defect abundant Si nanorods for high-performance Li-ion battery anodes. ACS Nano 9, 6576–6586 (2015). https://doi.org/10.1021/acsnano.5b02565
Jiang, H., Zhou, X., Liu, G., et al.: Free-standing Si/Graphene paper using Si nanoparticles synthesized by acid-etching Al–Si Alloy powder for high-stability Li-ion battery anodes. Electrochim. Acta 188, 777–784 (2016). https://doi.org/10.1016/j.electacta.2015.12.023
Zhang, S., Zheng, Y., Huang, X., et al.: Structural engineering of hierarchical micro-nanostructured Ge-C framework by controlling the nucleation for ultralong-life Li storage. Adv. Energy Mater. 9, 1900081 (2019). https://doi.org/10.1002/aenm.201900081
Wang, B., Jin, J., Rui, K., et al.: Scalable synthesis of hierarchical porous Ge/rGO microspheres with an ultra-long cycling life for lithium storage. J. Power Sources 396, 124–133 (2018). https://doi.org/10.1016/j.jpowsour.2018.06.024
Cui, G., Gu, L., Zhi, L., et al.: A Germanium-carbon nanocomposite material for lithium batteries. Adv. Mater. 20, 3079–3083 (2008). https://doi.org/10.1002/adma.200800586
Yang, H., Huang, S., Huang, X., et al.: Orientation-dependent interfacial mobility governs the anisotropic swelling in lithiated silicon nanowires. Nano Lett. 12, 1953–1958 (2012). https://doi.org/10.1021/nl204437t
Han, J., Li, C., Lu, Z., et al.: Vapor phase dealloying: a versatile approach for fabricating 3D porous materials. Acta Mater. 163, 161–172 (2019). https://doi.org/10.1016/j.actamat.2018.10.012
Liu, S., Feng, J., Bian, X., et al.: Nanoporous germanium as high-capacity lithium-ion battery anode. Nano Energy 13, 651–657 (2015). https://doi.org/10.1016/j.nanoen.2015.03.039
Hao, Q., Liu, Q., Zhang, Y., et al.: Easy preparation of nanoporous Ge/Cu3Ge composite and its high performances towards lithium storage. J. Colloid Interface Sci. 539, 665–671 (2019). https://doi.org/10.1016/j.jcis.2018.12.104
Hu, R.Z., Zhang, L., Liu, X., et al.: Investigation of immiscible alloy system of Al–Sn thin films as anodes for lithium ion batteries. Electrochem. Commun. 10, 1109–1112 (2008). https://doi.org/10.1016/j.elecom.2008.05.012
Chen, L.B., Xie, J.Y., Yu, H.C., et al.: Si–Al thin film anode material with superior cycle performance and rate capability for lithium ion batteries. Electrochim. Acta 53, 8149–8153 (2008). https://doi.org/10.1016/j.electacta.2008.06.025
Wang, M., Zhang, F., Lee, C.S., et al.: Low-cost metallic anode materials for high performance rechargeable batteries. Adv. Energy Mater. 7, 1700536 (2017). https://doi.org/10.1002/aenm.201700536
Ma, W., Wang, Y., Yang, Y., et al.: Temperature-dependent Li storage performance in nanoporous Cu–Ge–Al alloy. ACS Appl. Mater. Interfaces 11, 9073–9082 (2019). https://doi.org/10.1021/acsami.8b20654
Cook, J.B., Detsi, E., Liu, Y., et al.: Nanoporous tin with a granular hierarchical ligament morphology as a highly stable Li-ion battery anode. ACS Appl. Mater. Interfaces 9, 293–303 (2017). https://doi.org/10.1021/acsami.6b09014
Cook, J.B., Lin, T.C., Detsi, E., et al.: Using X-ray microscopy to understand how nanoporous materials can be used to reduce the large volume change in alloy anodes. Nano Lett. 17, 870–877 (2017). https://doi.org/10.1021/acs.nanolett.6b04181
Song, T., Yan, M., Qian, M.: A dealloying approach to synthesizing micro-sized porous tin (Sn) from immiscible alloy systems for potential lithium-ion battery anode applications. J. Porous Mater. 22, 713–719 (2015). https://doi.org/10.1007/s10934-015-9944-6
Reddy, M.V., Subba Rao, G.V., Chowdari, B.V.R.: Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 113, 5364–5457 (2013). https://doi.org/10.1021/cr3001884
Liu, J., Wen, Y., van Aken, P.A., et al.: Facile synthesis of highly porous Ni–Sn intermetallic microcages with excellent electrochemical performance for lithium and sodium storage. Nano Lett. 14, 6387–6392 (2014). https://doi.org/10.1021/nl5028606
Liu, X., Zhang, R., Yu, W., et al.: Three-dimensional electrode with conductive Cu framework for stable and fast Li-ion storage. Energy Storage Mater. 11, 83–90 (2018). https://doi.org/10.1016/j.ensm.2017.09.008
Xing, Y., Wang, S., Fang, B., et al.: Three-dimensional nanoporous Cu6Sn5/Cu composite from dealloying as anode for lithium ion batteries. Microporous Mesoporous Mater. 261, 237–243 (2018). https://doi.org/10.1016/j.micromeso.2016.11.036
He, M., Walter, M., Kravchyk, K.V., et al.: Monodisperse SnSb nanocrystals for Li-ion and Na-ion battery anodes: synergy and dissonance between Sn and Sb. Nanoscale 7, 455–459 (2015). https://doi.org/10.1039/C4NR05604C
Antitomaso, P., Fraisse, B., Sougrati, M.T., et al.: Ultra-fast dry microwave preparation of SnSb used as negative electrode material for Li-ion batteries. J. Power Sources 325, 346–350 (2016). https://doi.org/10.1016/j.jpowsour.2016.06.010
Zhang, J., Wang, Z., Hong, Y., et al.: Electrochemical fabrication of porous Sn/SnSb negative electrodes from mixed SnO2–Sb2O3. Electrochem. Commun. 38, 36–39 (2014). https://doi.org/10.1016/j.elecom.2013.10.030
Ding, Y., Li, Z.F., Timofeeva, E.V., et al.: In Situ EXAFS-derived mechanism of highly reversible tin phosphide/graphite composite anode for Li-ion batteries. Adv. Energy Mater. 8, 1702134 (2018). https://doi.org/10.1002/aenm.201702134
Xia, Y., Han, S., Zhu, Y., et al.: Stable cycling of mesoporous Sn4P3/SnO2@C nanosphere anode with high initial coulombic efficiency for Li-ion batteries. Energy Storage Mater. 18, 125–132 (2019). https://doi.org/10.1016/j.ensm.2019.01.021
Wang, X., Kim, H.M., Xiao, Y., et al.: Nanostructured metal phosphide-based materials for electrochemical energy storage. J. Mater. Chem. A 4, 14915–14931 (2016). https://doi.org/10.1039/C6TA06705K
Liu, Q., Ye, J., Chen, Z., et al.: Double conductivity-improved porous Sn/Sn4P3@carbon nanocomposite as high performance anode in lithium-ion batteries. J. Colloid Interface Sci. 537, 588–596 (2019). https://doi.org/10.1016/j.jcis.2018.11.060
Xing, Z., Ju, Z., Yang, J., et al.: One-step hydrothermal synthesis of ZnFe2O4 nano-octahedrons as a high capacity anode material for Li-ion batteries. Nano Res. 5, 477–485 (2012). https://doi.org/10.1007/s12274-012-0233-2
Xing, Z., Ji, X., Zhao, Y., et al.: Co2+xTi1−xO4 nano-octahedra as high performance anodes for lithium-ion batteries. J. Mater. Chem. A 5, 8714–8724 (2017). https://doi.org/10.1039/C7TA01152K
Tan, C., Cao, J., Khattak, A.M., et al.: High-performance tin oxide-nitrogen doped graphene aerogel hybrids as anode materials for lithium-ion batteries. J. Power Sources 270, 28–33 (2014). https://doi.org/10.1016/j.jpowsour.2014.07.059
Mei, J., Liao, T., Kou, L., et al.: Two-dimensional metal oxide nanomaterials for next-generation rechargeable batteries. Adv. Mater. 29, 1700176 (2017). https://doi.org/10.1002/adma.201700176
Xu, C., Wang, R., Zhang, Y., et al.: A general corrosion route to nanostructured metal oxides. Nanoscale 2, 906–909 (2010). https://doi.org/10.1039/B9NR00351G
Jia, S., Song, T., Zhao, B., et al.: Dealloyed Fe3O4 octahedra as anode material for lithium-ion batteries with stable and high electrochemical performance. J. Alloys Compd. 617, 787–791 (2014). https://doi.org/10.1016/j.jallcom.2014.08.081
Hao, Q., Wang, Z., Ye, J., et al.: Fe3O4/Ag microsheets assembled by interlaced nanothorns as high performance anode materials for lithium storage. Int. J. Hydrogen Energy 42, 10072–10080 (2017). https://doi.org/10.1016/j.ijhydene.2017.01.079
Ye, J., Wang, Z., Hao, Q., et al.: Facile fabrication of Fe3O4 octahedra/nanoporous copper network composite for high-performance anode in Li-ion batteries. J. Colloid Interface Sci. 493, 171–180 (2017). https://doi.org/10.1016/j.jcis.2017.01.036
Li, X., Huang, X., Liu, D., et al.: Synthesis of 3D hierarchical Fe3O4/graphene composites with high lithium storage capacity and for controlled drug delivery. J. Phys. Chem. C 115, 21567–21573 (2011). https://doi.org/10.1021/jp204502n
Ye, J., Hao, Q., Liu, B., et al.: Facile preparation of graphene nanosheets encapsulated Fe3O4 octahedra composite and its high lithium storage performances. Chem. Eng. J. 315, 115–123 (2017). https://doi.org/10.1016/j.cej.2017.01.023
Hao, Q., Ye, J., Xu, C.: Facile fabrication of Fe3O4 octahedra with bimodal conductive network of nanoporous Cu and graphene nanosheets for high-performance anode in Li-ion batteries. J. Alloys Compd. 727, 34–42 (2017). https://doi.org/10.1016/j.jallcom.2017.08.139
Hao, Q., Wang, J., Xu, C.: Facile preparation of Mn3O4 octahedra and their long-term cycle life as an anode material for Li-ion batteries. J. Mater. Chem. A 2, 87–93 (2014). https://doi.org/10.1039/C3TA13510A
Hao, Q., Liu, B., Ye, J., et al.: Well encapsulated Mn3O4 octahedra in graphene nanosheets with much enhanced Li-storage performances. J. Colloid Interface Sci. 504, 603–610 (2017). https://doi.org/10.1016/j.jcis.2017.05.079
Liu, B., Qi, L., Ye, J., et al.: Facile fabrication of graphene-encapsulated Mn3O4 octahedra cross-linked with a silver network as a high-capacity anode material for lithium ion batteries. New J. Chem. 41, 13454–13461 (2017). https://doi.org/10.1039/C7NJ03498A
Jiang, X., Wang, Y., Yang, L., et al.: Dealloying to porous hybrid manganese oxides microspheres for high performance anodes in lithium ion batteries. J. Power Sources 274, 862–868 (2015). https://doi.org/10.1016/j.jpowsour.2014.10.088
Hao, Q., Li, M., Jia, S., et al.: Controllable preparation of Co3O4 nanosheets and their electrochemical performance for Li-ion batteries. RSC Adv. 3, 7850–7854 (2013). https://doi.org/10.1039/C3RA23448G
Hao, Q., Yu, Y., Zhao, D., et al.: Composited Co3O4/Ag with flower-like nanosheets anchored on a porous substrate as a high-performance anode for Li-ion batteries. J. Mater. Chem. A 3, 15944–15950 (2015). https://doi.org/10.1039/C5TA03658E
Liang, C., Gao, M., Pan, H., et al.: Lithium alloys and metal oxides as high-capacity anode materials for lithium-ion batteries. J. Alloys Compd. 575, 246–256 (2013). https://doi.org/10.1016/j.jallcom.2013.04.001
Hao, Q., Chen, L., Xu, C.: Facile fabrication of a three-dimensional cross-linking TiO2 nanowire network and its long-term cycling life for lithium storage. ACS Appl. Mater. Interfaces 6, 10107–10112 (2014). https://doi.org/10.1021/am5010305
Zhao, Y., Wang, L.P., Sougrati, M.T., et al.: A review on design strategies for carbon based metal oxides and sulfides nanocomposites for high performance Li and Na ion battery anodes. Adv. Energy Mater. 7, 1601424 (2017). https://doi.org/10.1002/aenm.201601424
Ye, J., Zhao, D., Hao, Q., et al.: Facile fabrication of hierarchical manganese-cobalt mixed oxide microspheres as high-performance anode material for lithium storage. Electrochim. Acta 222, 1402–1409 (2016). https://doi.org/10.1016/j.electacta.2016.11.117
Wang, Z., Fei, P., Xiong, H., et al.: CoFe2O4 nanoplates synthesized by dealloying method as high performance Li-ion battery anodes. Electrochim. Acta 252, 295–305 (2017). https://doi.org/10.1016/j.electacta.2017.08.189
Hao, Q., Zhao, D., Duan, H., et al.: Porous Co3O4/CuO composite assembled from nanosheets as high-performance anodes for lithium-ion batteries. ChemSusChem 8, 1435–1441 (2015). https://doi.org/10.1002/cssc.201403420
Zhao, W., Fei, P., Zhang, X., et al.: Porous TiO2/Fe2O3 nanoplate composites prepared by de-alloying method for Li-ion batteries. Mater. Lett. 211, 254–257 (2018). https://doi.org/10.1016/j.matlet.2017.10.019
Zhao, D., Hao, Q., Xu, C.: Nanoporous TiO2/Co3O4composite as an anode material for lithium-ion batteries. Electrochim. Acta 211, 83–91 (2016). https://doi.org/10.1016/j.electacta.2016.06.043
Ye, J., Hao, Q., Xu, C.: Facile preparation of nanoporous TiO2/MoOx composite and its high lithium storage performances as an anode material. Int. J. Hydrogen Energy 42, 6820–6828 (2017). https://doi.org/10.1016/j.ijhydene.2016.12.077
Zhao, D., Hao, Q., Xu, C.: Facile fabrication of composited Mn3O4/Fe3O4 nanoflowers with high electrochemical performance as anode material for lithium ion batteries. Electrochim. Acta 180, 493–500 (2015). https://doi.org/10.1016/j.electacta.2015.08.146
Xu, H., Wang, X., Liu, H., et al.: Facile synthesis of Fe3O4/NiFe2O4 nanosheets with enhanced lithium-ion storage by one-step chemical dealloying. J. Mater. Sci. 53, 15631–15642 (2018). https://doi.org/10.1007/s10853-018-2729-y
Wang, Z., Zhang, X., Yan, Y., et al.: Nanoporous GeO2/Cu/Cu2O network synthesized by dealloying method for stable Li-ion storage. Electrochim. Acta 300, 363–372 (2019). https://doi.org/10.1016/j.electacta.2019.01.127
Liu, H., Wang, X., Wang, J., et al.: Hierarchical porous CoNi/CoO/NiO composites derived from dealloyed quasicrystals as advanced anodes for lithium-ion batteries. Scripta Mater. 139, 30–33 (2017). https://doi.org/10.1016/j.scriptamat.2017.06.011
Liu, H., Wang, X., Xu, H., et al.: Nanostructured CoO/NiO/CoNi anodes with tunable morphology for high performance lithium-ion batteries. Dalton Trans. 46, 11031–11036 (2017). https://doi.org/10.1039/C7DT01904A
Zhang, R., Wang, Y., Jia, M., et al.: One-pot hydrothermal synthesis of ZnS quantum dots/graphene hybrids as a dual anode for sodium ion and lithium ion batteries. Appl. Surf. Sci. 437, 375–383 (2018). https://doi.org/10.1016/j.apsusc.2017.12.110
Wang, Z., Zhang, X., Zhang, Y., et al.: Chemical dealloying synthesis of CuS nanowire-on-nanoplate network as anode materials for Li-ion batteries. Metals 8, 252–261 (2018). https://doi.org/10.3390/met8040252
Liu, Q., Chen, Z., Qin, R., et al.: Hierarchical mulberry-like Fe3S4/Co9S8 nanoparticles as highly reversible anode for lithium-ion batteries. Electrochim. Acta 304, 405–414 (2019). https://doi.org/10.1016/j.electacta.2019.03.034
Zielasek, V., Jürgens, B., Schulz, C., et al.: Gold catalysts: nanoporous gold foams. Angew. Chem. Int. Ed. 45, 8241–8244 (2006). https://doi.org/10.1002/anie.200602484
Yu, Y., Yan, C., Gu, L., et al.: Three-dimensional (3D) bicontinuous Au/amorphous-Ge thin films as fast and high-capacity anodes for lithium-ion batteries. Adv. Energy Mater. 3, 281–285 (2013). https://doi.org/10.1002/aenm.201200496
Guo, X., Han, J., Zhang, L., et al.: A nanoporous metal recuperated MnO2 anode for lithium ion batteries. Nanoscale 7, 15111–15116 (2015). https://doi.org/10.1039/C5NR05011A
Ye, J., Baumgaertel, A.C., Wang, Y.M., et al.: Structural optimization of 3D porous electrodes for high-rate performance lithium ion batteries. ACS Nano 9, 2194–2202 (2015). https://doi.org/10.1021/nn505490u
Liu, W., Chen, X., Xiang, P., et al.: Chemically monodisperse tin nanoparticles on monolithic 3D nanoporous copper for lithium ion battery anodes with ultralong cycle life and stable lithium storage properties. Nanoscale 11, 4885–4894 (2019). https://doi.org/10.1039/C8NR09398A
Dong, X., Liu, W., Chen, X., et al.: Novel three dimensional hierarchical porous Sn–Ni alloys as anode for lithium ion batteries with long cycle life by pulse electrodeposition. Chem. Eng. J. 350, 791–798 (2018). https://doi.org/10.1016/j.cej.2018.06.031
Liu, W., Zhang, S., Li, N., et al.: Preparation and characterization of sandwich-typed three-dimensional nanoporous copper-supported tin thin-film anode for lithium ion battery. Int. J. Electrochem. Sci. 8, 347–358 (2013)
Luo, Z., Xu, J.C., Yuan, B., et al.: A novel 3D bimodal porous current collector with large interconnected spherical channels for improved capacity and cycling stability of Sn anode in Li-ion batteries. Mater. Lett. 213, 189–192 (2018). https://doi.org/10.1016/j.matlet.2017.11.089
Luo, Z., Xu, J., Yuan, B., et al.: 3D hierarchical porous Cu-based composite current collector with enhanced ligaments for notably improved cycle stability of Sn anode in Li-ion batteries. ACS Appl. Mater. Interfaces 10, 22050–22058 (2018). https://doi.org/10.1021/acsami.8b04049
Han, G., Um, J.H., Park, H., et al.: Hierarchically structured nanoporous copper for use as lithium-ion battery anode. Scripta Mater. 163, 9–13 (2019). https://doi.org/10.1016/j.scriptamat.2018.12.030
Hou, C., Lang, X.Y., Han, G.F., et al.: Integrated solid/nanoporous copper/oxide hybrid bulk electrodes for high-performance lithium-ion batteries. Sci. Rep. 3, 2878 (2013). https://doi.org/10.1038/srep02878
Liu, Y., Xiong, L., Li, P., et al.: Self-supported CuO nanoflake arrays on nanoporous Cu substrate as high-performance negative-electrodes for lithium-ion batteries. J. Power Sources 428, 20–26 (2019). https://doi.org/10.1016/j.jpowsour.2019.04.102
Xu, X., Han, M., Ma, J., et al.: Preparation of a nanoporous CuO/Cu composite using a dealloy method for high performance lithium-ion batteries. RSC Adv. 5, 71760–71764 (2015). https://doi.org/10.1039/C5RA14123K
Yang, W., Wang, J., Ma, W., et al.: Free-standing CuO nanoflake arrays coated Cu foam for advanced lithium ion battery anodes. J. Power Sources 333, 88–98 (2016). https://doi.org/10.1016/j.jpowsour.2016.09.154
Wang, X., Liu, D., Weng, Q., et al.: Cu/Li4Ti5O12 scaffolds as superior anodes for lithium-ion batteries. NPG Asia Mater. 7, e171–e171 (2015). https://doi.org/10.1038/am.2015.23
Rahman, M.A., Zhu, X., Wen, C.: Fabrication of nanoporous Ni by chemical dealloying Al from Ni–Al alloys for lithium-ion batteries. Int. J. Electrochem. Sci. 10, 3767–3783 (2015)
Li, Y.Q., Li, J.C., Lang, X.Y., et al.: Lithium ion breathable electrodes with 3D hierarchical architecture for ultrastable and high-capacity lithium storage. Adv. Funct. Mater. 27, 1700447 (2017). https://doi.org/10.1002/adfm.201700447
Han, J., Liu, P., Ito, Y., et al.: Bilayered nanoporous graphene/molybdenum oxide for high rate lithium ion batteries. Nano Energy 45, 273–279 (2018). https://doi.org/10.1016/j.nanoen.2018.01.006
Han, J., Hirata, A., Du, J., et al.: Intercalation pseudocapacitance of amorphous titanium dioxide@nanoporous graphene for high-rate and large-capacity energy storage. Nano Energy 49, 354–362 (2018). https://doi.org/10.1016/j.nanoen.2018.04.063
Cheng, X.B., Zhao, C.Z., Yao, Y.X., et al.: Recent advances in energy chemistry between solid-state electrolyte and safe lithium-metal anodes. Chem 5, 74–96 (2019). https://doi.org/10.1016/j.chempr.2018.12.002
Peng, H.J., Huang, J.Q., Cheng, X.B., et al.: Review on high-loading and high-energy lithium-sulfur batteries. Adv. Energy. Mater. 7, 1700260 (2017). https://doi.org/10.1002/aenm.201700260
Lu, J., Li, L., Park, J.-B., et al.: Aprotic and aqueous Li-O2batteries. Chem. Rev. 114, 5611–5640 (2014). https://doi.org/10.1021/cr400573b
Balaish, M., Jung, J.W., Kim, I.D., et al.: A critical review on functionalization of air-cathodes for nonaqueous Li–O2batteries. Adv. Funct. Mater. (2019). https://doi.org/10.1002/adfm.201808303
Armand, M., Tarascon, J.M.: Building better batteries. Nature 451, 652–657 (2008). https://doi.org/10.1038/451652a
Liu, Y., Li, B., Kitaura, H., et al.: Fabrication and performance of all-solid-state Li-air battery with SWCNTs/LAGP cathode. ACS Appl. Mater. Interfaces 7, 17307–17310 (2015). https://doi.org/10.1021/acsami.5b04409
Xia, C., Kwok, C.Y., Nazar, L.F.: A high-energy-density lithium-oxygen battery based on a reversible four-electron conversion to lithium oxide. Science 361, 777–781 (2018). https://doi.org/10.1126/science.aas9343
Abraham, K.M., Jiang, Z.: A polymer electrolyt-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 143, 1–5 (1996). https://doi.org/10.1149/1.1836378
Zhang, W., Huang, Y., Liu, Y., et al.: Strategies toward stable nonaqueous alkali metal-O2 batteries. Adv. Energy Mater. 9, 1900464 (2019). https://doi.org/10.1002/aenm.201900464
Peng, Z., Freunberger, S.A., Chen, Y., et al.: A reversible and higher-rate Li-O2 battery. Science 337, 563–566 (2012). https://doi.org/10.1126/science.1223985
Chen, Y., Freunberger, S.A., Peng, Z., et al.: Charging a Li–O2 battery using a redox mediator. Nat. Chem. 5, 489–494 (2013). https://doi.org/10.1038/nchem.1646
Gittleson, F.S., Ryu, W.H., Taylor, A.D.: Operando observation of the gold-electrolyte interface in Li–O2 batteries. ACS Appl. Mater. Interfaces 6, 19017–19025 (2014). https://doi.org/10.1021/am504900k
Wen, R., Byon, H.R.: In situ monitoring of the Li–O2 electrochemical reaction on nanoporous gold using electrochemical AFM. Chem. Commun. 50, 2628–2631 (2014). https://doi.org/10.1039/C3CC49625B
Chen, L.Y., Guo, X.W., Han, J.H., et al.: Nanoporous metal/oxide hybrid materials for rechargeable lithium-oxygen batteries. J. Mater. Chem. A 3, 3620–3626 (2015). https://doi.org/10.1039/C4TA05738D
Yang, H., Xia, J., Bromberg, L., et al.: Electrochemically synthesized nanoporous gold as a cathode material for Li–O2 batteries. J. Solid State Electrochem. 21, 463–468 (2017). https://doi.org/10.1007/s10008-016-3374-5
Guo, X., Han, J., Liu, P., et al.: Graphene@nanoporous nickel cathode for Li-O2 batteries. ChemNanoMat 2, 176–181 (2016). https://doi.org/10.1002/cnma.201500214
Zhao, G., Zhang, L., Niu, Y., et al.: Enhanced durability of Li–O2 batteries employing vertically standing Ti nanowire array supported cathodes. J. Mater. Chem. A 4, 4009–4014 (2016). https://doi.org/10.1039/C6TA00318D
Zhao, G., Zhang, L., Niu, Y., et al.: A molten Mg corrosion method for preparing porous Ti foam as self-supported Li–O2battery cathodes. Electrochim. Acta 224, 64–70 (2017). https://doi.org/10.1016/j.electacta.2016.12.033
Lim, H.D., Lee, B., Bae, Y., et al.: Reaction chemistry in rechargeable Li–O2 batteries. Chem. Soc. Rev. 46, 2873–2888 (2017). https://doi.org/10.1039/C6CS00929H
Surwade, S.P., Smirnov, S.N., Vlassiouk, I.V., et al.: Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 10, 459–464 (2015). https://doi.org/10.1038/nnano.2015.37
Ito, Y., Cong, W., Fujita, T., et al.: High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew. Chem. Int. Ed. 54, 2131–2136 (2015). https://doi.org/10.1002/anie.201410050
Moreno, C., Vilas-Varela, M., Kretz, B., et al.: Bottom-up synthesis of multifunctional nanoporous graphene. Science 360, 199–203 (2018). https://doi.org/10.1126/science.aar2009
Han, J., Guo, X., Ito, Y., et al.: Effect of chemical doping on cathodic performance of bicontinuous nanoporous graphene for Li–O2 batteries. Adv. Energy Mater. 6, 1501870 (2016). https://doi.org/10.1002/aenm.201501870
Park, J.B., Lee, S.H., Jung, H.G., et al.: Redox mediators for Li–O2 batteries: status and perspectives. Adv. Mater. 30, 1704162 (2018). https://doi.org/10.1002/adma.201704162
Han, J., Huang, G., Ito, Y., et al.: Full performance nanoporous graphene based Li–O2batteries through solution phase oxygen reduction and redox-additive mediated Li2O2 oxidation. Adv. Energy Mater. 7, 1601933 (2017). https://doi.org/10.1002/aenm.201601933
Guo, X., Liu, P., Han, J., et al.: 3D nanoporous nitrogen-doped graphene with encapsulated RuO2 nanoparticles for Li–O2 batteries. Adv. Mater. 27, 6137–6143 (2015). https://doi.org/10.1002/adma.201503182
Ding, Y., Chen, M., Erlebacher, J.: Metallic mesoporous nanocomposites for electrocatalysis. J. Am. Chem. Soc. 126, 6876–6877 (2004). https://doi.org/10.1021/ja0320119
Wang, R., Wang, C., Cai, W.-B., et al.: Ultralow-platinum-loading high-performance nanoporous electrocatalysts with nanoengineered surface structures. Adv. Mater. 22, 1845–1848 (2010). https://doi.org/10.1002/adma.200903548
Li, J., Yin, H.M., Li, X.B., et al.: Surface evolution of a Pt–Pd–Au electrocatalyst for stable oxygen reduction. Nat. Energy 2, 1–9 (2017). https://doi.org/10.1038/nenergy.2017.111
Manthiram, A., Fu, Y., Su, Y.-S.: Challenges and prospects of lithiu-sulfur batteries. Acc. Chem. Res. 46, 1125–1134 (2013). https://doi.org/10.1021/ar300179v
Ji, X., Nazar, L.F.: Advances in Li-S batteries. J. Mater. Chem. 20, 9821–9826 (2010). https://doi.org/10.1039/B925751A
Ji, X., Lee, K.T., Nazar, L.F.: A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 8, 500–506 (2009). https://doi.org/10.1038/nmat2460
Seh, Z.W., Sun, Y., Zhang, Q., et al.: Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 45, 5605–5634 (2016). https://doi.org/10.1039/C5CS00410A
He, G., Ji, X., Nazar, L.: High “C” rate Li-S cathodes: sulfur imbibed bimodal porous carbons. Energy Environ. Sci. 4, 2878–2883 (2011). https://doi.org/10.1039/C1EE01219C
He, J., Luo, L., Chen, Y., et al.: Yolk-shelled C@Fe3O4nanoboxes as efficient sulfur hosts for high-performance lithium-sulfur batteries. Adv. Mater. 29, 1702707 (2017). https://doi.org/10.1002/adma.201702707
Lu, L.Q., Schriever, N., De Hosson, J.T.M., et al.: Low-temperature solid-state growth of three-dimensional bicontinuous nanoporous graphene with tunable porosity for lithium-sulfur batteries. J. Mater. Chem. A 6, 11405–11415 (2018). https://doi.org/10.1039/C8TA03488E
Gu, X., Tong, C.J., Lai, C., et al.: A porous nitrogen and phosphorous dual doped graphene blocking layer for high performance Li-S batteries. J. Mater. Chem. A 3, 16670–16678 (2015). https://doi.org/10.1039/C5TA04255K
Zhou, G., Zhao, Y., Manthiram, A.: Dual-confined flexible sulfur cathodes encapsulated in nitrogen-doped double-shelled hollow carbon spheres and wrapped with graphene for Li-S batteries. Adv. Energy Mater. 5, 1402263 (2015). https://doi.org/10.1002/aenm.201402263
Shi, J.L., Tang, C., Huang, J.Q., et al.: Effective exposure of nitrogen heteroatoms in 3D porous graphene framework for oxygen reduction reaction and lithium-sulfur batteries. J. Energy Chem. 27, 167–175 (2018). https://doi.org/10.1016/j.jechem.2017.09.014
Liu, N., Wang, L., Zhao, Y., et al.: Hierarchically porous TiO2 matrix encapsulated sulfur and polysulfides for high performance lithium/sulfur batteries. J. Alloys Compd. 769, 678–685 (2018). https://doi.org/10.1016/j.jallcom.2018.08.027
Wu, L., Wang, Z., An, C., et al.: Chemical-dealloying to fabricate nonconductive interlayers for high-loading lithium sulfur batteries. J. Alloys Compd. 806, 881–888 (2019). https://doi.org/10.1016/j.jallcom.2019.07.339
Zhao, Y., Tian, Y., Zhang, X., et al.: Mn3O4 octahedral microparticles prepared by facile dealloying process as efficient sulfur hosts for lithium/sulfur batteries. Metal 8, 515–522 (2018). https://doi.org/10.3390/met8070515
Lin, D., Liu, Y., Cui, Y.: Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017). https://doi.org/10.1038/nnano.2017.16
Barai, P., Higa, K., Srinivasan, V.: Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies. Phys. Chem. Chem. Phys. 19, 20493–20505 (2017). https://doi.org/10.1039/C7CP03304D
Jin, S., Jiang, Y., Ji, H., et al.: Advanced 3D current collectors for lithium-based batteries. Adv. Mater. 30, 1802014 (2018). https://doi.org/10.1002/adma.201802014
Rosso, M., Brissot, C., Teyssot, A., et al.: Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 51, 5334–5340 (2006). https://doi.org/10.1016/j.electacta.2006.02.004
Yun, Q., He, Y.B., Lv, W., et al.: Chemical dealloying derived 3D porous current collector for Li metal anodes. Adv. Mater. 28, 6932–6939 (2016). https://doi.org/10.1002/adma.201601409
Zhao, H., Lei, D., He, Y.B., et al.: Compact 3D copper with uniform porous structure derived by electrochemical dealloying as dendrite-free lithium metal anode current collector. Adv. Energy Mater. 8, 1800266 (2018). https://doi.org/10.1002/aenm.201800266
An, Y., Fei, H., Zeng, G., et al.: Vacuum distillation derived 3D porous current collector for stable lithium-metal batteries. Nano Energy 47, 503–511 (2018). https://doi.org/10.1016/j.nanoen.2018.03.036
Liu, H., Wang, E., Zhang, Q., et al.: Unique 3D nanoporous/macroporous structure Cu current collector for dendrite-free lithium deposition. Energy Storage Mater. 17, 253–259 (2019). https://doi.org/10.1016/j.ensm.2018.07.010
Shi, Y., Wang, Z., Gao, H., et al.: A self-supported, three-dimensional porous copper film as a current collector for advanced lithium metal batteries. J. Mater. Chem. A 7, 1092–1098 (2019). https://doi.org/10.1039/C8TA09384A
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2019YFA0205700), the National Natural Science Foundation of China (51602219, 51671145), the National Science Fund for Distinguished Young Scholars (51825102), the joint research fund of NSFC (51761165012) and the Macau Science and Technology Fund (FDCT, 002/2017/AFJ), and the Tianjin Municipal Science and Technology Commission (17JCYBJC42000). X.W. and G.H. contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., He, G. & Ding, Y. Dealloyed nanoporous materials for rechargeable lithium batteries. Electrochem. Energ. Rev. 3, 541–580 (2020). https://doi.org/10.1007/s41918-020-00070-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-020-00070-7