Abstract
The sleep–wake cycle is a process regulated by multiple neurobiological mechanisms that in aberrant functioning provokes several sleep disturbances. Among the major categories of sleep disorders, insomnia represents one of the most reported in population. Pharmacological interventions aimed for treating this sleep disturbance include compounds such as antidepressants, antihistamines, sedative-hypnotics, among others. However, using pharmacological treatments increase undesirable side effects such as addiction to sleep-inducing drugs. Here, we review and summarize recent publications available in PubMed regarding the use of non-pharmacological/invasive means to control insomnia, including physical exercise and transcranial direct current stimulation (tDCS). Current data suggest that these two strategies efficiently manage insomnia, and in turn opens new approaches to develop therapeutical tools to diminish this pathology. Nevertheless, additional research is required to understand the neurobiological mechanism of action of physical exercise and tDCS in insomnia control.

Similar content being viewed by others
References
American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic coding manual, 3rd ed. Darien, IL, USA: American Academy of Sleep Medicine; 2014
Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22:S347–53.
Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307–49.
Monti JM, Torterolo P, Perumal SRP. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51–7.
Araújo T, Jarrin DC, Leanza Y, Vallières A, Morin CM. Qualitative studies of insomnia: current state of knowledge in the field. Sleep Med Rev. 2017;31:58–69.
Doufas AG, Panagiotou OA, Panousis P, Wong SS, Ioannidis JPA. Insomnia from drug treatments: evidence from meta-analyses of randomized trials and concordance with prescribing information. Mayo Clin Proc. 2017;92:72–87.
van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62.
Sadler P, McLaren S, Klein B, Jenkins M. Advancing cognitive behaviour therapy for older adults with comorbid insomnia and depression. Cogn Behav Ther. 2017;8:1–16.
Vitiello MV. Cognitive-behavioural therapy for insomnia is effective, safe and highly deployable. Evid Based Nurs. 2017;20:92.
van Maanen A, Meijer AM, Smits MG, van der Heijden KB, Oort FJ. Effects of melatonin and bright light treatment in childhood chronic sleep onset insomnia with late melatonin onset: a randomized controlled study. Sleep. 2017;40(2). https://doi.org/10.1093/sleep/zsw038.
Yeung WF, Ho FY, Chung KF, Zhang ZJ, Yu BY, Suen LK, Chan LY, Chen HY, Ho LM, Lao LX. Self-administered acupressure for insomnia disorder: a pilot randomized controlled trial. J Sleep Res. 2017 (In press).
Hayhoe S. Insomnia: can acupuncture help? Pain Manag. 2017;7:49–57.
Zhou ES, Gardiner P, Bertisch SM. Integrative medicine for insomnia. Med Clin North Am. 2017;101:865–79.
Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29:409–12.
Chen LJ, Steptoe A, Chen YH, Ku PW, Lin CH. Physical activity, smoking, and the incidence of clinically diagnosed insomnia. Sleep Med. 2017;30:189–94.
Morita Y, Sasai-Sakuma T, Inoue Y. Effects of acute morning and evening exercise on subjective and objective sleep quality in older individuals with insomnia. Sleep Med. 2017;34:200–8.
Gerber M, Brand S, Holsboer-Trachsler E, Pühse U. Fitness and exercise as correlates of sleep complaints: is it all in our minds? Med Sci Sports Exerc. 2010;42:893–901.
Spörndly-Nees S, Åsenlöf P, Lindberg E. High or increasing levels of physical activity protect women from future insomnia. Sleep Med. 2017;32:22–7.
Dzierzewski JM, Buman MP, Giacobbi PR, Roberts BL, Aiken-Morgan AT, Marsiske M, McCrae CS. Exercise and sleep in community-dwelling older adults: evidence for a reciprocal relationship. J Sleep Res. 2014;23:61–8.
Montgomery PI, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002;(4):CD003404. https://doi.org/10.1002/14651858.CD003404.
Horne JA, Staff LHE. Exercise and sleep: body-heating effects. Sleep. 1983;6:36–46.
Passos GS, Poyares DLR, Santana MG, Tufik S, Mello MT. Is exercise an alternative treatment for chronic insomnia? Clinics. 2012;67:653–60.
Passos GS, Poyares D, Santana MG, Tufik S, de Mello MT. Effect of chronic physical exercise on anxiety in patients with chronic primary insomnia. Sleep Med. 2009;10:S19.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;2:126–31.
Brand S, Kalak N, Gerber M, Kirov R, Pühse U, Holsboer-Trachsler E. High self-perceived exercise exertion before bedtime is associated with greater objectively assessed sleep efficiency. Sleep Med. 2014;15:1031–6.
Bonato M, La Torre A, Saresella M, Marventano I, Merati G, Vitale JA. Salivary cortisol concentration after high-intensity interval exercise: time of day and chronotype effect. Chronobiol Int. 2017;34:698–707.
Budde H, Machado S, Ribeiro P, Wegner M. The cortisol response to exercise in young adults. Front Behav Neurosci. 2015;9:13.
Chen G, Xia L, Wang F, Li X, Jiao C. Patients with chronic insomnia have selective impairments in memory that are modulated by cortisol. Psychophysiology. 2016;53:1567–76.
Passos GS, Poyares D, Santana MG, Teixeira AADS, Lira FS, Youngstedt SD, Santos RVT, Tufik S, De Mello MT. Exercise improves immune function, antidepressive response, and sleep quality in patients with chronic primary insomnia. Biomed Res Int. 2014;2014:1–7.
Lack LC, Gradisar M, Van Someren EJW, Wright HR, Lushington K. The relationship between insomnia and body temperatures. Sleep Med Rev. 2008;12:307–17.
Cai ZY, Chen KWC, Wen HJ. Effects of a group-based step aerobics training on sleep quality and melatonin levels in sleep-impaired postmenopausal women. J Strength Cond Res. 2014;28:2597–603.
Kim HJ, Kim DH. Effect of different exercise intensity on blood melatonin density in sleep disordered rats. J Korean Soc Phys Med. 2014;9:45–53.
Atkinson G, Edwards B, Reilly T, Waterhouse J. Exercise as a synchroniser of human circadian rhythms: an update and discussion of the methodological problems. Eur J Appl Physiol. 2007;99:331–41.
Escames G, Ozturk G, Baño-Otálora B, Pozo MJ, Madrid JA, Reiter RJ, Serrano E, Concepción M, Acuña-Castroviejo D. Exercise and melatonin in humans: reciprocal benefits. J Pineal Res. 2012;52:1–11.
Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210.
Cover H, Irwin M. Immunity and depression: insomnia, retardation, and reduction of natural killer cell activity. J Behav Med. 1994;17:217–23.
Nehlsen-Cannarella SL, Nieman DC, Balk-Lamberton AJ, Markoff PA, Chritton DB, Gusewitch G, Lee JW. The effects of moderate exercise training on immune response. Med Sci Sports Exerc. 1991;23:64–70.
Guilleminault C, Clerk A, Black J, Labanowski M, Pelayo R, Claman D. Nondrug treatment trials in psychophysiologic insomnia. Arch Intern Med. 1995;155:838–44.
Chen LJ, Fox KR, Ku PW, Chang YW. Effects of aquatic exercise on sleep in older adults with mild sleep impairment: a randomized controlled trial. Int J Behav Med. 2016;23:501–6.
Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58:157–63.
Hartescu I, Morgan K, Stevinson CD. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: a randomized controlled trial. J Sleep Res. 2015;24:526–34.
Rouleau CR, Horsley KJ, Morse E, Aggarwal S, Bacon SL, Campbell TS. The Association between insomnia symptoms and mood changes during exercise among patients enrolled in cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2015;35:409–16.
Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38:427–49.
Mendelson M, Borowik A, Michallet A, Perrin C, Monneret D, Faure P, Levy P, Pépin J, Wuyam B, Flore P. Sleep quality, sleep duration and physical activity in obese adolescents: effects of exercise training. Pediatr Obes. 2016;11:26–32.
Schmitt A, Falkai P. Aerobic exercise in major psychiatric disorders: promises and challenges. Eur Arch Psychiatry Clin Neurosci. 2017;267:93–4.
Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11:934–40.
Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4.
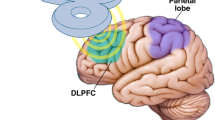
Lattari E, Costa SS, Campos C, De Oliveira AJ, Machado S, Neto GAM. Can transcranial direct current stimulation on the dorsolateral prefrontal cortex improves balance and functional mobility in Parkinson’s disease? Neurosci Lett. 2017;63:6165–9.
Dondé C, Amad A, Nieto I, Brunoni AR, Neufeld NH, Bellivier F, Poulet E, Geoffroy PA. Transcranial direct-current stimulation (tDCS) for bipolar depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:123–31.
Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174:628–39.
Göbel CH, Tronnier VM, Münte TF. Brain stimulation in obesity. Int J Obes. 2017 (In press).
ALHarbi MF, Armijo-Olivo S, Kim ES. Transcranial direct current stimulation (tDCS) to improve naming ability in post-stroke aphasia: a critical review. Behav Brain Res. 2017;332:7–15.
Weinberger AB, Green AE, Chrysikou EG. Using transcranial direct current stimulation to enhance creative cognition: interactions between task, polarity, and stimulation site. Front Hum Neurosci. 2017;11:246.
Minichino A, Bersani FS, Spagnoli F, Corrado A, De Michele F, Calò WK, Primavera M, Yang B, Bernabei L, Macrì F. Prefronto-cerebellar transcranial direct current stimulation improves sleep quality in euthymic bipolar patients: a brief report. Behav Neurol. 2014;2014:876521.
McKenna BS, Eyler LT. Overlapping prefrontal systems involved in cognitive and emotional processing in euthymic bipolar disorder and following sleep deprivation: a review of functional neuroimaging studies. Clin Psychol Rev. 2012;32:650–63.
DelRosso LM, Hoque R. The cerebellum and sleep. Neurol Clin. 2014;32:893–900.
Galbiati A, Abutalebi J, Iannaccone S, Borsa VM, Musteata S, Zucconi M, Giora E, Ferini-Strambi L. The effects of transcranial direct current stimulation (tDCS) on idiopathic hypersomnia: a pilot study. Arch Ital Biol. 2016;154:1–5.
de Sá AS, Campos C, Rocha NB, Yuan TF, Paes F, Arias-Carrión O, Carta MG, Nardi AE, Cheniaux E, Machado S. Neurobiology of bipolar disorder: abnormalities on cognitive and cortical functioning and biomarker levels. CNS Neurol Disord Targets. 2016;15:713–22.
Pedroso JL, Braga-Neto P, Felício AC, Aquino CCH, Prado LB, Prado GFD, Barsottini OGP. Sleep disorders in cerebellar ataxias. Arq Neuropsiquiatr. 2011;69:253–7.
Saebipour MR, Joghataei MT, Yoonessi A, Sadeghniiat-Haghighi K, Khalighinejad N, Khademi S. Slow oscillating transcranial direct current stimulation during sleep has a sleep-stabilizing effect in chronic insomnia: a pilot study. J Sleep Res. 2015;24:518–25.
Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci. 2008;105:15160–5.
Kaufmann C, Wehrle R, Wetter TC, Holsboer F, Auer DP, Pollmächer T, Czisch M. Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study. Brain. 2005;129:655–67.
Morairty SR, Dittrich L, Pasumarthi RK, Valladao D, Heiss JE, Gerashchenko D, Kilduff TS. A role for cortical nNOS/NK1 neurons in coupling homeostatic sleep drive to EEG slow wave activity. Proc Natl Acad Sci. 2013;110:20272–7.
Gerashchenko D, Wisor JP, Kilduff TS. Sleep-active cells in the cerebral cortex and their role in slow-wave activity. Sleep Biol Rhythms. 2011;9:71–7.
Maness DL, Khan M. Nonpharmacologic management of chronic insomnia. Am Fam Physician. 2015;92:1058–64.
Hollenbach D, Broker R, Herlehy S, Stuber K. Non-pharmacological interventions for sleep quality and insomnia during pregnancy: a systematic review. J Can Chiropr Assoc. 2013;57:260–70.
Dolezal BA, Neufeld EV, Boland DM, Martin JL, Cooper CB. Interrelationship between sleep and exercise: a systematic review. Adv Prev Med. 2017;2017:1364387.
Milne S, Elkins MR. Exercise as an alternative treatment for chronic insomnia (PEDro synthesis). Br J Sports Med. 2017;51:479–80.
Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504.
Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, Espie CA, Garcia-Borreguero D, Gjerstad M, Gonçalves M, Hertenstein E, Jansson-Fröjmark M, Jennum PJ, Leger D, Nissen C, Parrino L, Paunio T, Pevernagie D, Verbraecken J, Weeß HG, Wichniak A, Zavalko I, Arnardottir ES, Deleanu OC, Strazisar B, Zoetmulder M, Spiegelhalder K. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017 (In press).
Funding
This work was supported by The University of California Institute for Mexico and the United States (UC MEXUS) and Consejo Nacional de Ciencia y Tecnología (CONACyT. Grant CN-17-19) and Escuela de Medicina, Universidad Anáhuac Mayab Grant (PresInvEMR2017) given to EM-R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All data reported in this paper are from public repositories.
Conflict of interest
Authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Higuera-Hernández, M.F., Reyes-Cuapio, E., Gutiérrez-Mendoza, M. et al. Alternative Strategies for Managing Insomnia: The Case of Physical Exercise and Transcranial Direct Current Stimulation. A Narrative Review. Sleep Vigilance 2, 39–44 (2018). https://doi.org/10.1007/s41782-018-0037-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41782-018-0037-x