Abstract
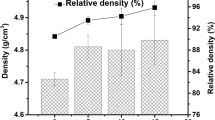
At present, the formation conditions of cordierite were relatively harsh, the formation temperature was close to the decomposition temperature, and it was difficult to control the sintering temperature. In order to solve the problem of cordierite synthesis, cordierite powders were synthesized by chemical coprecipitation–rotation evaporation and solid reaction sintering. The thermodynamics, thermal analysis, phase composition, specific surface area, morphology, and grain size distribution of the samples sintered at different temperatures were systematically studied. SEM micrographs showed that the crystal structural evolution of cordierite was divided into three processes; the temperatures of 1000 °C (the formation of hexagonal flake–like grain) and 1200 °C (the formation of flaky texture grain) were two important sintering transition points. The specific surface area of the sample sintered at 1400 °C was 1.76 m2/g, and the grain size was well developed with the range of 196.5–222.7 nm. Phase analysis revealed that the main crystal phase of samples sintered at 1200 °C was cordierite phase and a little spinel phase and mullite phase, and the peak intensity of the characteristic peaks at 1400 °C was more intense.









Similar content being viewed by others
References
Kobayashi, Y., Katayama, M., Kato, M., Kuramochi, S.: Effect on microstructure on the thermal expansion coefficient of sintered cordierite prepared from sol mixtures. J. Am. Ceram. Soc. 96(6), 1863–1868 (2013)
Sembiring, S., Simanjuntak, W., Situmeang, R., Riyanto, A., Sebayang, K.: Preparation of refractory cordierite using amorphous rice husk silica for thermal insulation purposes. Ceram. Int. 42, 8431–8437 (2016)
Kuscer, D., Bantan, I., Hrovat, M., Mali, B.: The microstructure, coefficient of thermal expansion and flexural strength of cordierite ceramics prepared from alumina with different particle sizes. J. Eur. Ceram. Soc. 37, 739–746 (2017)
Kolli, M., Hamidouche, M., Fantozzi, G., Chevalier, J.: Elaboration and characterization of a refractory based on Algerian kaolin. Ceram. Int. 33, 1435–1443 (2007)
Orosco, P., Del, M., Ruiz, C., González, J.: Synthesis of cordierite by dolomite and kaolinitic clay chlorination. Study of the phase transformations and reaction mechanism. Powder Technol. 267, 111–118 (2014)
Shaohong, W., Haoran, L., Zhaoxia, H.: Sol-emulsion-gel synthesis of cordierite ceramics for high-frequency multilayer chip inductors. Ceram. Int. 36, 991–997 (2013)
Janos, R., Lazau, I., Pacurariu, C.: Solution combustion synthesis of α-cordierite. J. Alloys Compd. 480, 702–705 (2009)
Menchi, A.M., Scian, A.N.: Mechanism of cordierite formation obtained by the sol–gel technique. Mater. Lett. 59, 2664–2667 (2005)
Cheraghi, A., Shoushtarizadeh, H., Malekfar, R., Alizadeh, O.: Synthesis of <alpha>-cordierite nanoparticles from bentonite using thermal shock assisted solid-state reaction method. Adv. Powder Technol. 29, 124–129 (2018)
El-Buaishi, N.M., Veljović, D., Jokić, B., Zeljko, R., Steins, I., Janać ković, D., Petrović, R.: Conventional and spark-plasma sintering of cordierite powders synthesized by sol–gel methods. Ceram. Int. 39, 5845–5854 (2013)
de Almeida, E.P., de Brito, I.P., Ferreira, H.C., de Lucena Lira, H., de Lima Santana, L.N., de Araújo Neves, G.: Cordierite obtained from compositions containing kaolin waste, talc and magnesium oxide. Ceram. Int. 44, 1719–1725 (2018)
Kuscer, D., Bantan, I., Hrovat, M., Malί, B.: The microstructure, coefficient of thermal expansion and flexural strength of cordierite ceramics prepared from alumina with different particle sizes. J. Eur. Ceram. Soc. 37, 739–746 (2017)
Zhang, L., Wu, Y., Zhang, L., Wang, Y., Li, M.: Synthesis and characterization of mesoporous alumina with high specific area via coprecipitation method. Vacuum. 133, 1–6 (2016)
Menchi, A., Scian, A.: Mechanism of cordierite formation obtained by the sol–gel technique. Mater. Lett. 59, 2664–2667 (2005)
Liu, Y., Liu, H., Zhang, Z., Lu, H., Chen, Y.: In-situ Hydrothermal synthesis of ZSM-5/cordierite monolith catalyst. J. Chin. Ceram. Soc. 43, 926–933 (2015)
Wang, S., Lu, H., Zhaoxia, H., Hu, X., Changlei, N., Xue, Z., Wang, H., Wang, C.: Effect of Bi2O3 on sintering and dielectric properties of cordierite ceramics synthesized by sol-emulsion-gel technique. Rare Metal Mater. Eng. 40, 360–363 (2011)
Potdar, H.S., Vijayanand, S., Mohaideen, K.K., et al.: A simple chemical co-precipitation/calcination route for the synthesis of simulated synroc-B and synroc-C powders. Mater. Chem. Phys. 123, 695–699 (2010)
Najafi, A.: Development of high oxidation resistant coating of nanostructured MgO on carbon nanotubes via simple precipitation technique in Mg/CO gas system. Ceram. Int. 42, 18573–18578 (2016)
Nguyen, T.A., Tr Nguyen, L.T., Bui, V.X., et al.: Optical and magnetic properties of HoFeO3 nanocrystals prepared by a simple co-precipitation method using ethanol. J. Alloys Compd. 8345, 155098 (2020)
Castro-Rodríguez, R., Mendez-Gamboa, J., Perez-Quintana, I., Medina-Ezquivel, R.: CdS thin films growth by fast evaporation with substrate rotation. Appl. Surf. Sci. 257, 9480–9484 (2011)
Zhang, B., Ma, B., Zhu, Q., et al.: In-situ formation and densification of MgAl2O4-Y3Al5O12 and MgAl2O4-MgNb2O6 ceramics via a single-stage SRS process. Sci. Sinter. 49(3), 285–297 (2017)
Ma, B., Chang, S., Ren, X., et al.: Preparation and properties of porous mullite ceramics with high-closed porosity and high strength from fly ash via reaction synthesis process. J. Alloys Compd. 803, 981–991 (2019)
Chen, Z.: Chemical thermodynamics of refractories [M], pp. 642–655. Metallurgical Industry Press, Beijing (2005)
Yuan, L., Ma, B., Yulong, D., et al.: Characterization and properties of ZrO2-forsterite composites from zircon and magnesite via reaction sintering. J. Ceram. Process. Res. 18(5), 352–356 (2017)
Luo, X., Dianli, Q., Zhang, G., et al.: Structure characterization of cordierite synthesized from decomposed magnesite pyrophyllite. Chinese J. Inorg. Chem. 27(3), 434–438 (2011)
Acimovic, Z., Pavlovic, L., Trumbulovic, L., Andric, L., Stamatovic, M.: Synthesis and characterization of the cordierite ceramics from nonstandard raw materials for application in foundry. Mater. Lett. 57, 2651–2656 (2003)
Zhou, J., Xia, Z., Chen, M., Molokeev, M.S., Liu, Q.: New insight into phase formation of MxMg2Al4+xSi5xO18:Eu2+ solid solution phosphors and its luminescence properties. Sci. Rep. 5, 12149 (2015)
Funding
The work is supported by National Natural Science Foundation of China (51772139).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hou, Q., Luo, X., Liu, X. et al. Preparation of cordierite powder by chemical coprecipitation–rotation evaporation and solid reaction sintering. J Aust Ceram Soc 56, 1575–1582 (2020). https://doi.org/10.1007/s41779-020-00496-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-020-00496-8