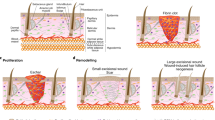
Abstract
Skin, being the protective barrier against the environment, can be subject to frequent trauma and stress, hence has the ability to heal itself rapidly. This capacity is attributed to a large number of resident stem cells and progenitors in the skin, which are activated to proliferate, migrate and differentiate to recreate the cellular diversity and regain tissue integrity. The barrier function of the skin is maintained by the epidermis, a multilayered epithelial compartment formed of keratinocytes. Wound repair evokes the capacity of cells to sense and respond to environmental cues. Tissue damage demands rapid cellular action, wherein spatio-temporally regulated cellular responses are critical determinants of the outcome of healing. Hence cells surrounding the wound have to immediately sense the damage and must activate the key signaling pathways to launch the wound-healing response. Emerging data is indicating that mechanical tension release is one of the first cues sensed by the neighboring cells of the damage. This cue is relayed by the cytoskeleton and converted into biochemical and cellular signals, which help the cells to respond accordingly to the trauma. In this review, we will focus on the role of keratinocytes and keratinocyte stem cells in wound healing, and the cytoskeletal dynamics involved therein.


Similar content being viewed by others
References
Roshan A, Jones PH (2012) Act your age: tuning cell behavior to tissue requirements in interfollicular epidermis. Semin Cell Dev Biol 23:884–889
Fuchs E (2007) Scratching the surface of skin development. Nature 445:834–842
Jacob JT, Coulombe PA, Kwan R, Omary MB (2018) Types I and II keratin intermediate filaments. Cold Spring Harb Perspect Biol 10:a018275
Sanghvi-Shah R, Weber GF (2017) Intermediate filaments at the junction of mechanotransduction, migration, and development. Front Cell Dev Biol 5:1–19
Zhang X, Yin M, Zhang LJ (2019) Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells 8:1–14
Fuchs E (2008) Skin stem cells: rising to the surface. J Cell Biol 180:273–284
Delva E, Tucker DK, Kowalczyk AP (2009) The desmosome. Cold Spring Harbor Perspect Biol 1:a002543
Tsuruta D, Hashimoto T, Hamill KJ, Jones JCR (2011) Hemidesmosomes and focal contact proteins: functions and cross-talk in keratinocytes, bullous diseases and wound healing. J Dermatol Sci 62:1–7
Karsch S, Büchau F, Magin TM, Janshoff A (2020) An intact keratin network is crucial for mechanical integrity and barrier function in keratinocyte cell sheets. Cell Mol Life Sci. https://doi.org/10.1007/s00018-019-03424-7
Blanpain C, Fuchs E (2006) Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22:339–373
Blanpain C, Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10:207–217
Sumigray K, Lechler T (2015) Cell adhesion in epidermal development and barrier formation. Curr Top Dev Biol 112:383–414
Cotsarelis G (2006) Epithelial stem cells: a folliculocentric view. J Invest Dermatol 126:1459–1468
Vidal VPI et al (2005) Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol 15:1340–1351
Adam RC et al (2015) Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature 521:366–370
Rhee H, Polak L, Fuchs E (2006) Lhx2 maintains stem cell character in hair follicles. Science 312:1946–1949
Plikus MV et al (2012) Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol 23:946–953
Ito M et al (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11:1351–1354
Folgueras AR et al (2013) Architectural Niche Organization by LHX2 is linked to hair follicle stem cell function. Cell Stem Cell 13:314–327
Mardaryev AN et al (2011) Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development 138:4843–4852
Gurtner G, Werner S, Barrandon Y, Longaker M (2008) Wound repair and regeneration. Nature 453:314–321
Pastar I et al (2014) Epithelialization in wound healing: a comprehensive review. Adv Wound Care 3:445–464
Sommerlad BC, Creasey JM (1978) The stretched scar: a clinical and histological study. Br J Plast Surg 31:34–45
Gomez GA, McLachlan RW, Yap AS (2011) Productive tension: force-sensing and homeostasis of cell–cell junctions. Trends Cell Biol 21:499–505
Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260:1124–1127
Reichelt J (2007) Mechanotransduction of keratinocytes in culture and in the epidermis. Eur J Cell Biol 86:807–816
Sun Z, Guo SS, Fässler R (2016) Integrin-mediated mechanotransduction. J Cell Biol 215:445–456
Wang W et al (2020) Hemidesmosomes modulate force generation via focal adhesions. J Cell Biol 219:1–19
Russell D, Andrews PD, James J, Lane EB (2004) Mechanical stress induces profound remodelling of keratin filaments and cell junctions in epidermolysis bullosa simplex keratinocytes. J Cell Sci 117:5233–5243
Cheah JS, Jacobs KA, Heinrich V, Lo SH, Yamada S (2019) Force-induced recruitment of cten along keratin network in epithelial cells. Proc Natl Acad Sci USA 116:19799–19801
Kenny FN et al (2018) Tissue stiffening promotes keratinocyte proliferation via activation of epidermal growth factor signaling. J Cell Sci 131(10):jcs215780
Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K (2004) Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. J Invest Dermatol 122:783–790
Takei T et al (1997) Effect of strain on human keratinocytes in vitro. J Cell Physiol 173:64–72
Dupont S et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179–184
Lee MJ, Byun MR, Furutani-Seiki M, Hong JH, Jung HS (2014) YAP and TAZ regulate skin wound healing. J Invest Dermatol 134:518–525
Fan R, Kim NG, Gumbiner BM (2013) Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA 110:2569–2574
Beverdam A et al (2013) Yap controls stem/progenitor cell proliferation in the mouse postnatal epidermis. J Invest Dermatol 133:1497–1505
Zhang H, Pasolli HA, Fuchs E (2011) Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci USA 108:2270–2275
Eckert RL, Welter JF (1996) Transcription factor regulation of epidermal keratinocyte gene expression. Mol Biol Rep 23:59–70
Zenz R et al (2008) Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthr Res Ther 10:201
Yang M et al (2011) A preliminary study of differentially expressed genes in expanded skin and normal skin: implications for adult skin regeneration. Arch Dermatol Res 303:125–133
Park S et al (2017) Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat Cell Biol 19:155–163
Aragona M et al (2017) Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun 8:1–14
Safferling K et al (2013) Wound healing revised: a novel reepithelialization mechanism revealed by in vitro and in silico models. J Cell Biol 203:691–709
Haensel D, Dai X (2018) Epithelial-to-mesenchymal transition in cutaneous wound healing: where we are and where we are heading. Dev Dyn 247:473–480
Hudson LG et al (2009) Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2). J Dermatol Sci 56:19–26
Komine M et al (2000) Inflammatory versus proliferative processes epidermis. Tumor necrosis factor α induces K6b keratin synthesis through a transcriptional complex containing NFκB and C/EBPβ. J Biol Chem 275:32077–32088
Jiang CK et al (1993) Epidermal growth factor and transforming growth factor α specifically induce the activation- and hyperproliferation-associated keratins 6 and 16. Proc Natl Acad Sci USA 90:6786–6790
Takahashi K, Yan B, Yamanishi K, Imamura S, Coulombe PA (1998) The two functional keratin 6 genes of mouse are differentially regulated and evolved independently from their human orthologs. Genomics 53:170–183
Hatzfeld M, Keil R, Magin TM (2017) Desmosomes and intermediate filaments: their consequences for tissue mechanics. Cold Spring Harb Perspect Biol 9:a029157
Thomason HA et al (2012) Direct evidence that PKCá positively regulates wound re-epithelialization: correlation with changes in desmosomal adhesiveness. J Pathol 227:346–356
Santoro MM, Gaudino G (2005) Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res 304:274–286
Serrels B et al (2007) Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol 9:1046–1056
Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9:858–867
Kuwahara M, Hatoko M, Tada H, Tanaka A (2001) E-cadherin expression in wound healing of mouse skin. J Cutan Pathol 28:191–199
Seetharaman S, Etienne-Manneville S (2020) Cytoskeletal crosstalk in cell migration. Trends Cell Biol 30:720–735
Charafeddine RA, Nosanchuk JD, Sharp DJ (2016) Targeting microtubules for wound repair. Adv Wound Care 5:444–454
Zhang J et al (2019) Microtubule-associated protein 4 phosphorylation regulates epidermal keratinocyte migration and proliferation. Int J Biol Sci 15:1962–1976
Schmitt S et al (2013) Stathmin regulates keratinocyte proliferation and migration during cutaneous regeneration. PLoS ONE 8:1–15
Charafeddine RA et al (2015) Fidgetin-like 2: a microtubule-based regulator of wound healing. J Invest Dermatol 135:2309–2318
Suozzi KC, Wu X, Fuchs E (2012) Spectraplakins: master orchestrators of cytoskeletal dynamics. J Cell Biol 197:465–475
Wu X, Kodama A, Fuchs E (2008) ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell 135:137–148
Wu X et al (2011) Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3β. Cell 144:341–352
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Kalekar LA, Rosenblum MD (2019) Regulatory T cells in inflammatory skin disease: from mice to humans. Int Immunol 31:457–463
Hassan WU, Greiser U, Wang W (2014) Role of adipose-derived stem cells in wound healing. Wound Repair Regen 22:313–325
DiPietro LA (2016) Angiogenesis and wound repair: when enough is enough. J Leukoc Biol 100:979–984
Schäfer M, Werner S (2008) Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 9:628–638
Sundaram GM, Quah S, Sampath P (2018) Cancer: the dark side of wound healing. FEBS J 285:4516–4534
Arwert EN, Hoste E, Watt FM (2012) Epithelial stem cells, wound healing and cancer. Nat Rev Cancer 12:170–180
Stojadinovic O et al (2005) Molecular pathogenesis of chronic wounds: the role of β-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol 167:59–69
Acknowledgements
A.H. and A. SHP A. are supported by the Council of Scientific and Industrial Research, India. S.G. is supported by the Early Career Fellowship from The Wellcome Trust - DBT India Alliance. C.K. has been generously hosted by the laboraotory of Prof. Colin Jamora, inStem, Bangalore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hegde, A., Ananthan, A.S., Kashyap, C. et al. Wound Healing by Keratinocytes: A Cytoskeletal Perspective. J Indian Inst Sci 101, 73–80 (2021). https://doi.org/10.1007/s41745-020-00219-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-020-00219-9