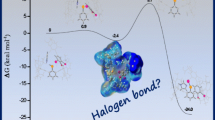
Abstract
Halogen bonding (XB) is an attractive interaction between a halogen atom and an electron donor. Although halogens are electron-rich atoms, they act as electrophiles in these types of interactions. This is due to the presence of a significant positive charge (σ-hole) on the halogen atoms in organic halides along the R-X (R = carbon, nitrogen, halogen) bond. With an increase in the polarizability down the group from fluorine to iodine, the positive charge on the σ-hole increases, which leads to an increase in the strength of XB. Numerous studies revealed that XB is a useful tool to develop supramolecular architectures by self-assembly. Interestingly, XBs are also observed in many biomolecules, such as protein–ligand complexes and nucleic acids containing halogenated nucleotides. In fact, XBs are extensively used to increase the potency and selectivity of small molecule ligands to a target protein. In this minireview, we discuss the role of XBs in the molecular recognition of thyroid hormones (THs) and their metabolites by various transport proteins and thyroid hormone receptors (TRs). THs are naturally occurring iodinated small molecules that are synthesized by the thyroid gland and carried to various target organs by several serum transport proteins, such as transthyretin, human serum albumin, and thyroxine-binding globulin. Interestingly, all these proteins form XBs with THs and these interactions play important roles in the high affinity binding. Furthermore, TRs, such as TRα and TRβ also form XBs with the 3-iodine of THs and triiodothyroacetic acid, an endogenous TH metabolite that shows thyromimetic activity.







Similar content being viewed by others
References
Metrangolo P, Resnati G (2013) Metal-bound halogen atoms in crystal engineering. Chem Commun 49:1783–1785
Nakamoto K, Margoshes M, Rundle RE (1955) Stretching frequencies as a function of distances in hydrogen bonds. J Am Chem Soc 77:6480–6486
Von P, Schleyer R, West R (1959) Comparison of covalently bonded electro-negative atoms as proton acceptor groups in hydrogen bonding. J Am Chem Soc 81:3164–3165
Colin M (1814) Note Sur Quelques Combinaisons de L′iode. Ann Chim 91:252–272
Pelletier JCP (1819) Sur Un Nouvel Alcali Végétal (la Strychine) Trouvé Dans La Fève de Saint-Ignace, La Noix Vomique Etc., Ann Chim Phys 10:142–177
P. N. 2009-032-1-100 (2010) Categorizing halogen bonding and other noncovalent interactions involving halogen atoms. Chem Int 32:20 − 21
Desiraju GRH, Ho PS, Kloo L, Legon AC, Marquardt R, Metrangolo P, Politzer P, Resnati G, Rissanen K (2013) Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl Chem 85:1711–1713
Clark T, Hennemann M, Murray JS, Politzer P (2007) Halogen bonding: the sigma-hole. In: Proceedings of “Modeling interactions in biomolecules II”, Prague, 5th–9th September 2005, J Mol Model 13:291–296
Murray JS, Lane P, Clark T, Politzer P (2007) Sigma-hole bonding: molecules containing group VI atoms. J Mol Model 13:1033–1038
Auffinger P, Hays FA, Westhof E, Ho PS (2004) Halogen bonds in biological molecules. Proc Natl Acad Sci USA 101:16789–16794
Valerio G, Raos G, Meille SV, Metrangolo P, Resnati G (2000) Halogen bonding in fluoroalkylhalides: a quantum chemical study of increasing fluorine substitution. J Phys Chem A 104:1617–1620
Gao K, Goroff NS (2000) Two New iodine-capped carbon rods. J Am Chem Soc 122:9320–9321
Sun A, Lauher JW, Goroff NS (2006) Preparation of poly(diiododiacetylene), an ordered conjugated polymer of carbon and iodine. Science 312:1030–1034
Zou JW, Jiang YJ, Guo M, Hu GX, Zhang B, Liu HC, Yu QS (2005) Ab initio study of the complexes of halogen-containing molecules RX (X = Cl, Br, and I) and NH3: towards understanding the nature of halogen bonding and the electron-accepting propensities of covalently bonded halogen atoms. Chem Eur J 11:740–751
Ananthavel SP, Manoharan M (2001) A theoretical study on electron donor–acceptor complexes of Et2O, Et2S and Me3N with interhalogens, I-X (X = Cl and Br). Chem Phys 269:49–57
Cavallo G, Metrangolo P, Milani R, Pilati T, Priimagi A, Resnati G, Terraneo G (2016) The halogen bond. Chem Rev 116:2478–2601
Gilday LC, Robinson SW, Barendt TA, Langton MJ, Mullaney BR, Beer PD (2015) Halogen bonding in supramolecular chemistry. Chem Rev 115:7118–7195
Metrangolo P, Meyer F, Pilati T, Resnati G, Terraneo G (2008) Halogen bonding in supramolecular chemistry. Angew Chem Int Ed 47:6114–6127
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Halogen bonding based recognition processes: a world parallel to hydrogen bonding. Acc Chem Res 38:386–395
Ford MC, Ho PS (2016) Computational tools to model halogen bonds in medicinal chemistry. J Med Chem 59:1655–1670
Hays FA, Teegarden A, Jones ZJ, Harms M, Raup D, Watson J, Cavaliere E, Ho PS (2005) How sequence defines structure: a crystallographic map of DNA structure and conformation. Proc Natl Acad Sci USA 102:7157–7162
Hays FA, Vargason JM, Ho PS (2003) Effect of sequence on the conformation of DNA holliday junctions. Biochemistry 42:9586–9597
Mendez L, Henriquez G, Sirimulla S, Narayan M (2017) Looking back, looking forward at halogen bonding in drug discovery. Molecules 22:1397
Parisini E, Metrangolo P, Pilati T, Resnati G, Terraneo G (2011) Halogen bonding in halocarbon-protein complexes: a structural survey. Chem Soc Rev 40:2267–2278
Wilcken R, Zimmermann MO, Lange A, Joerger AC, Boeckler FM (2013) Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J Med Chem 56:1363–1388
Mondal S, Gong X, Zhang X, Salinger AJ, Zheng L, Sen S, Weerapana E, Thompson PR (2019) Halogen bonding increases the potency and isozyme selectivity of protein arginine deiminase 1 Inhibitors. Angew Chem Int Ed 58:12476–12480
Jakka SR, Govindaraj V, Mugesh G (2019) A Single Atom Change Facilitates the Membrane Transport of Green Fluorescent Proteins in Mammalian Cells. Angew Chem Int Ed 58:7713–7717
Ungati H, Govindaraj V, Mugesh G (2018) The remarkable effect of halogen substitution on the membrane transport of fluorescent molecules in living cells. Angew Chem Int Ed 57:8989–8993
Ungati H, Govindaraj V, Nair CR, Mugesh G (2019) Halogen-mediated membrane transport: an efficient strategy for the enhancement of cellular uptake of synthetic molecules. Chem Eur J 25:3391–3399
Lu Y, Wang Y, Zhu W (2010) Nonbonding interactions of organic halogens in biological systems: implications for drug discovery and biomolecular design. Phys Chem Chem Phys 12:4543–4551
Brent GA (2012) Mechanisms of thyroid hormone action. J Clin Invest 122:3035–3043
Fekete C, Lechan RM (2007) Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Front Neuroendocrinol 28:97–114
Lechan RM, Fekete C (2004) Feedback regulation of thyrotropin-releasing hormone (TRH): mechanisms for the non-thyroidal illness syndrome. J Endocrinol Invest 27:105–119
Mullur R, Liu YY, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382
Mondal S, Raja K, Schweizer U, Mugesh G (2016) Chemistry and biology in the biosynthesis and action of thyroid hormones. Angew Chem Int Ed 55:7606–7630
Schussler GC (2000) The thyroxine-binding proteins. Thyroid 10:141–149
Berry MJ, Banu L, Larsen PR (1991) Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 349:438–440
Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89
Kohrle J (2002) Iodothyronine deiodinases. Methods Enzymol 347:125–167
Schweizer U, Steegborn C (2015) New insights into the structure and mechanism of iodothyronine deiodinases. J Mol Endocrinol 55:R37–R52
Davis PJ, Goglia F, Leonard JL (2016) Nongenomic actions of thyroid hormone. Nat Rev Endocrinol 12:111–121
Yen PM (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142
Eisele YS, Monteiro C, Fearns C, Encalada SE, Wiseman RL, Powers ET, Kelly JW (2015) Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discov 14:759–780
Wojtczak AC, Cody V, Luft JR, Pangborn W (1996) Structures of human transthyretin complexed with thyroxine at 2.0 Å resolution and 3′,5′-dinitro-N-acetyl-l-thyronine at 2.2 Å resolution. Acta Crystallogr Sect D 52:758–765
Eneqvist T, Lundberg E, Karlsson A, Huang S, Santos CR, Power DM, Sauer-Eriksson AE (2004) High resolution crystal structures of piscine transthyretin reveal different binding modes for triiodothyronine and thyroxine. J Biol Chem 279:26411–26416
Wojtczak A, Luft J, Cody V (1992) Mechanism of molecular recognition. Structural aspects of 3,3′-diiodo-l-thyronine binding to human serum transthyretin. J Biol Chem 267:353–357
Wojtczak AN, Neumann P, Cody V (2001) Structure of a new polymorphic monoclinic form of human transthyretin at 3 Å resolution reveals a mixed complex between unliganded and T4-bound tetramers of TTR. Acta Crystallogr Sect D 57:957–967
Hamilton JA, Steinrauf LK, Braden BC, Liepnieks J, Benson MD, Holmgren G, Sandgren O, Steen L (1993) The X-ray crystal structure refinements of normal human transthyretin and the amyloidogenic Val-30– > Met variant to 1.7-A resolution. J Biol Chem 268:2416–2424
Steinrauf LK, Hamilton JA, Braden BC, Murrell JR, Benson MD (1993) X-ray crystal structure of the Ala-109– > Thr variant of human transthyretin which produces euthyroid hyperthyroxinemia. J Biol Chem 268:2425–2430
Wojtczak AC, Cody V, Luft JR, Pangborn W (2001) Structure of rat transthyretin (rTTR) complex with thyroxine at 2.5 Å resolution: first non-biased insight into thyroxine binding reveals different hormone orientation in two binding sites. Acta Crystallogr Sect D 57:1061–1070
Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ (2005) Alternate pathways of thyroid hormone metabolism. Thyroid 15:943–958
Mondal S, Mugesh G (2017) Novel thyroid hormone analogues, enzyme inhibitors and mimetics, and their action. Mol Cell Endocrinol 458:91–104
Moreno M, De Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F (2008) Metabolic effects of thyroid hormone derivatives. Thyroid 18:239–253
Koehrle J, Auf′mkolk M, Rokos H, Hesch RD, Cody V (1986) Rat liver iodothyronine monodeiodinase. Evaluation of the iodothyronine ligand-binding site. J Biol Chem 261:11613–11622
Shepherdley CA, Klootwijk W, Makabe KW, Visser TJ, Kuiper GG (2004) An ascidian homolog of vertebrate iodothyronine deiodinases. Endocrinology 145:1255–1268
Neumann PC, Cody V, Wojtczak A (2005) Ligand binding at the transthyretin dimer-dimer interface: structure of the transthyretin-T4Ac complex at 2. Å resolution. Acta Crystallogr Sect D 61:1313–1319
Muziol TC, Cody V, Luft JR, Pangborn W, Wojtczak A (2001) Complex of rat transthyretin with tetraiodothyroacetic acid refined at 2.1 and 1.8 Å resolution. Acta Biochim Pol 48:877–884
Petitpas I, Petersen CE, Ha CE, Bhattacharya AA, Zunszain PA, Ghuman J, Bhagavan NV, Curry S (2003) Structural basis of albumin–thyroxine interactions and familial dysalbuminemic hyperthyroxinemia. Proc Natl Acad Sci USA 100:6440–6445
Mondal S, Mugesh G (2015) Structure elucidation and characterization of different thyroxine polymorphs. Angew Chem Int Ed 54:10833–10837
Mondal S, Mugesh G (2016) Conformational flexibility and halogen bonding in thyroid hormones and their metabolites. Cryst Growth Des 16:5896–5906
Zhou A, Wei Z, Read RJ, Carrell RW (2006) Structural mechanism for the carriage and release of thyroxine in the blood. Proc Natl Acad Sci USA 103:13321–13326
Qi X, Loiseau F, Chan WL, Yan Y, Wei Z, Milroy LG, Myers RM, Ley SV, Read RJ, Carrell RW, Zhou A (2011) Allosteric modulation of hormone release from thyroxine and corticosteroid-binding globulins. J Biol Chem 286:16163–16173
Evans RM (1988) The steroid and thyroid hormone receptor superfamily. Science 240:889–895
Nagy L, Schwabe JW (2004) Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci 29:317–324
Gullberg H, Rudling M, Salto C, Forrest D, Angelin B, Vennstrom B (2002) Requirement for thyroid hormone receptor beta in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol 16:1767–1777
Mittag J, Davis B, Vujovic M, Arner A, Vennstrom B (2010) Adaptations of the autonomous nervous system controlling heart rate are impaired by a mutant thyroid hormone receptor-alpha1. Endocrinology 151:2388–2395
Nascimento AS, Dias SM, Nunes FM, Aparicio R, Ambrosio AL, Bleicher L, Figueira AC, Santos MA, De Oliveira Neto M, Fischer H, Togashi M, Craievich AF, Garratt RC, Baxter JD, Webb P, Polikarpov I (2006) Structural rearrangements in the thyroid hormone receptor hinge domain and their putative role in the receptor function. J Mol Biol 360:586–598
Souza PC, Puhl AC, Martinez L, Aparicio R, Nascimento AS, Figueira AC, Nguyen P, Webb P, Skaf MS, Polikarpov I (2014) Identification of a new hormone-binding site on the surface of thyroid hormone receptor. Mol Endocrinol 28:534–545
Sandler B, Webb P, Apriletti JW, Huber BR, Togashi M, Cunha Lima ST, Juric S, Nilsson S, Wagner R, Fletterick RJ, Baxter JD (2004) Thyroxine-thyroid hormone receptor interactions. J Biol Chem 279:55801–55808
Martinez L, Nascimento AS, Nunes FM, Phillips K, Aparicio R, Dias SM, Figueira AC, Lin JH, Nguyen P, Apriletti JW, Neves FA, Baxter JD, Webb P, Skaf MS, Polikarpov I (2009) Gaining ligand selectivity in thyroid hormone receptors via entropy. Proc Natl Acad Sci USA 106:20717–20722
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mondal, S., Giri, D. & Mugesh, G. Halogen Bonding in the Molecular Recognition of Thyroid Hormones and Their Metabolites by Transport Proteins and Thyroid Hormone Receptors. J Indian Inst Sci 100, 231–247 (2020). https://doi.org/10.1007/s41745-019-00153-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-019-00153-5