Abstract
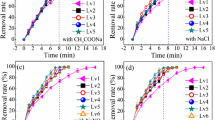
Chromium hydroxide is an important form present in chromium chemicals and a major product in the reduction of hexavalent chromium pollutants, and the study of chromium hydroxide re-oxidation process is crucial in controlling chromium pollution. The aim of this research was to investigate the re-oxidation performance of different forms of chromium hydroxide in air: crystalline chromium hydroxide (C-Cr(OH)3), amorphous chromium hydroxide (A-Cr(OH)3), chromium hydroxide obtained by reduction (R-Cr(OH)3), and aged R-Cr(OH)3 (Aged-R-Cr(OH)3). The results showed that A-Cr(OH)3 had the highest re-oxidation efficiency and the largest re-oxidation rate constant (k), followed by R-Cr(OH)3, Aged-R-Cr(OH)3, and C-Cr(OH)3. The study found that the re-oxidation rate of chromium hydroxide was mainly affected by the surface Cr–O bond energy and physical water. The advantageous re-oxidation of chromium hydroxide could be attributed to its diminutive bond energy of Cr–O and the presence of physical water on its surface. It was observed that increasing the temperature and adding salt (Na2SO4 and Na2CO4) promoted the re-oxidation of Cr(III) for different chromium hydroxides. This effect was particularly noticeable under alkaline conditions induced by Na2CO3 or at a reaction temperature of 200 °C. The re-oxidation rate constant of chromium hydroxides was up to 39.4 times higher at a reaction temperature of 200 °C than at 80 °C. This would be of great significance for chromium contamination removal by controlling the hexavalent chromium reduction products and environmental conditions.







Similar content being viewed by others
Data availability
Data included in the paper or supplementary material.
References
Ao M, Deng THB, Sun SS, Li MY, Li JJ, Liu T, Yan BF, Liu WS, Wang GB, Jing DD (2023) Increasing soil Mn abundance promotes the dissolution and oxidation of Cr (III) and increases the accumulation of Cr in rice grains. Environ Int 175:107939
Bai YK, Zheng RT, Gu Q, Wang JJ, Wang BS, Cheng GA, Chen G (2014) One-step synthesis of hollow Cr(OH)3 micro/nano-hexagonal pellets and the catalytic properties of hollow Cr2O3 structures. J Mater Chem A 2(32):12770–12775
Chen KY, Bocknek L, Manning B (2021) Oxidation of Cr(III) to Cr(VI) and production of Mn(II) by synthetic manganese(IV) oxide. Crystals 11:443
De Paris Junior O, Scapini T, Camargo AF, Venturin B, Dalastra C, Kubeneck S, Czapela FF, Preczeski KP, Stefanski FS, Korf EP, Valerio A, Di Luccio M, Mossi AJ, Fongaro G, Treichel H (2019) Removal of chromium from wastewater by swine hair residues applied as a putative biofilter. Environ Sci Pollut Res 26(32):33014–33022
Ding ZC, Sun GZ, Fu FL, Ye CJ (2022) Phase transformation of Cr(VI)-adsorbed ferr ihydr ite in the presence of Mn(II): fate of Mn(II) and Cr(VI). J Environ Sci 113:251–259
Fontaine M, Clement Y, Blanc N, Demesmay C (2019) Hexavalent chromium release from leather over time natural ageing vs accelerated ageing according to a multivariate approach. J Hazard Mater 368:811–818
Fu Y, Wang LL, Peng WY, Fan QY, Li QC, Dong YX, Liu YJ, Boczkaj G, Wang ZH (2021) Enabling simultaneous redox transformation of toxic chromium(VI) and arsenic(III) in aqueous media—a review. J Hazard Mater 417:126041
Hojjati-Najafabadi AH, Mozaffarinia R, Rahimi H, Razavi RS, Paimozd E (2013) Sol-gel processing of hybrid nanocomposite protective coatings using experimental design. Prog Org Coat 76:293–301
Hojjati-Najafabadi A, Aygun A, Tiri RNE, Gulbagca F, Lounissaa MI, Feng PZ, Karimi F, Sen F (2023a) Bacillus thuringiensis based ruthenium/nickel Co-doped zinc as a green nanocatalyst: enhanced photocatalytic activity, mechanism, and efficient H2 production from sodium borohydride methanolysis. Ind Eng Chem Res 62:4655–4664
Hojjati-Najafabadi A, Esfahani PN, Davar F, Aminabhavi TM, Vasseghian Y (2023b) Adsorptive removal of malachite green using novel GO@ZnO-NiFe2O4-αAl2O3 nanocomposites. Chem Eng J 471:144485
Huang ZL, Chen CG, Xie JY, Wang ZX (2016) The evolution of dehydration and thermal decomposition of nanocrystalline and amorphous chromium hydroxide. J Anal Appl Pyrol 118:225–230
Liu Q, Liu H, Chen H, Wang X, Hu D, Cheng X, Xu H (2020) Thermodynamic investigation with chemical kinetic analysis on the reoxidation phenomenon of the Cr(iii) in air. RSC Adv 10(46):27775–27787
Liu XL, Dong HL, Hansel CM (2021) Coupled Mn(II) and Cr(III) oxidation mediated by ascomycete fungi. Environ Sci Technol 55:16236–16245
Matveev VA, Kalinnikov VT, Zakharov VI, Maiorov DV (2011) Investigation of an effect exerted by methods of production of chromium(III) oxides-hydroxides on their physicochemical properties. Russ J Appl Chem 84(9):1524–1528
Mohanta S, Sahu MK, Mishra PC, Giri AK (2021) Removal of Cr (VI) from aqueous solution by activated charcoal derived from Sapindus trifoliate L fruit biomass using continuous fixed bed column studies. Water Sci Technol 84(1):55–65
Park JE, Shin JH, Oh W, Choi SJ, Kim J, Kim C, Jeon J (2022) Removal of hexavalent chromium(VI) from wastewater using chitosan-coated iron oxide nanocomposite membranes. Toxics 10(2):98
Qian A, Pan C, Yuan S, Giammar DE (2020) Cr(VI) formation from CrxFe1–x(OH)3 induced by Mn(II) oxidation on the surface of CrxFe1–x(OH)3. ACS Earth Space Chem 4(9):1558–1564
Qu M, Chen H, Wang Y, Wang X, Tong X, Li S, Xu H (2021) Improved performance and applicability of copper-iron bimetal by sulfidation for Cr(VI) removal. Chemosphere 281:130820
Shu Y, Ji B, Li Y, Zhang W, Zhang H, Zhang J (2021) Natural pyrite improved steel slag towards environmentally sustainable chromium reclamation from hexavalent chromium-containing wastewater. Chemosphere 282:130974
Singh V, Singh N, Verma M, Kamal R, Tiwari R, Chivate MS, Rai SN, Kumar A, Singh A, Singh MP, Vamanu E, Mishra V (2022) Hexavalent-chromium-induced oxidative stress and the protective role of antioxidants against cellular toxicity. Antioxidants 11(12):2375
Varadharajan C, Beller HR, Bill M, Brodie EL, Conrad ME, Han R, Irwin C, Larsen JT, Lim HC, Molins S, Steefel CI, van Hise A, Yang L, Nico PS (2017) Reoxidation of Chromium(III) products formed under different biogeochemical regimes. Environ Sci Technol 51(9):4918–4927
Wang ML, Risuleo RS, Jacobsen EW, Chotteau V, Hjalmarsson H (2020) Identification of nonlinear kinetics of macroscopic bio-reactions using multilinear Gaussian processes. Comput Chem Eng 133:106671
Xia CL, Joo SW, Hojjati-Najafabadi A, Xie H, Wu YJ, Mashifana T, Vasseghian Y (2023) Latest advances in layered covalent organic frameworks for water and wastewater treatment. Chemosphere 329:138580
Xu T, Jiang X, Tang Y, Zeng Y, Zhang W, Shi B (2021) Oxidation of trivalent chromium induced by unsaturated oils: a pathway for hexavalent chromium formation in soil. J Hazard Mater 405:124699
Yang HD, Zhao YP, Li SF, Fan X, Wei XY, Zong ZM (2016) Removal of hexavalent chromium from aqueous solution by calcined Zn/Al-LDHs. Water Sci Technol 74(1):229–235
Yao L, Shen Z, Ji Z, Hu Y, Tang D, Zhao G, Wang X (2022a) Cr(VI) detoxification and simultaneous selective recovery of Cr resource from wastewater via photo-chemical extraction using biomass. Sci Bull 67(21):2154–2157
Yao Y, Liu X, Hu H, Tang Y, Hu H, Ma Z, Wang S (2022b) Synthesis and characterization of iron-nitrogen-doped biochar catalysts for organic pollutant removal and hexavalent chromium reduction. J Colloid Interface Sci 610:334–346
Zhu Z, Miao Y, Wang G, Chen W, Lu W (2022) Solar-driven zinc-doped graphitic carbon nitride photocatalytic fibre for simultaneous removal of hexavalent chromium and pharmaceuticals. Environ Technol 43(17):2569–2580
Acknowledgements
This work were financially supported by National Natural Science Foundation of China (Grant No. 52000173) and Shijiazhuang High-level Science and Technology innovation and Entrepreneurship Talent Project (Project No. 06202102).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qu, M., Chen, H., Zhang, H. et al. Insight Into the Effects of Environmental and Structural Factors on the Re-Oxidation of Cr(III) Hydroxides. Int J Environ Res 18, 39 (2024). https://doi.org/10.1007/s41742-024-00581-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-024-00581-x