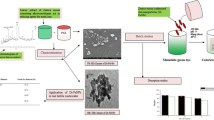
Abstract
Malachite Green removal from water using Layered Double Hydroxide/iron oxide nanoparticles as magnetic adsorbent was investigated due to the environmental risk that the difficulty of treatment and incorrect disposal of colored pollutants entail. Characterization, adsorption capacity, kinetics and equilibrium studies of the materials were carried out. The adsorbents reaching equilibrium showed MG adsorbed amounts (qe) of 198.94 and 172.13 mg g−1 for LDH and LDH/Iron oxides, respectively. Furthermore, for the three materials, in the kinetic study at low concentrations for metal oxides, a rapid adsorption was observed, with more than 80% of equilibrium reached in 200 min, while LDH took almost 400 min for the same performance. The composite kinetic behavior highlighted that the metallic oxides ensured that 80% adsorption was achieved below 200 min, and the pseudo-first order model was the one that best fitted for all materials. In addition, through the adsorption isotherms, a better fit to the Sips model was observed. Although LDH and the composite (LDH/Iron oxides) have shown excellent efficiency for pollutant removal, the difference lies in the adsorbent recovery process. LDH are well known as effective adsorbents for pollutant removal, but they have disadvantages linked to their mechanical properties and the costs related to material losses through the processes. The magnetic composite presented was able to improve the adsorbent material properties without losing its excellent adsorptive capacity, being a new competitive material against other adsorbents present in the current global market and showing superiority above 100% of removal when compared with biochar-LDH composites and isolated LDH's.
Graphical abstract

Highlights
-
iron magnetic LDH adsorbent was synthesized;
-
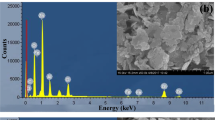
materials characterized by XRD, FTIR, RAMAN, EDS, BET, OM and SEM;
-
Superior adsorption features were observed;
-
A synergistic effect between the associated materials was achieved.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article.
References
Ahghari MR, Soltaninejad V, Maleki A (2020) Synthesis of nickel nanoparticles by a green and convenient method as a magnetic mirror with antibacterial activities. Sci Rep 2020(101):10. https://doi.org/10.1038/s41598-020-69679-4. (1–10)
Alderman DJ (1985) Malachite green: a review. J Fish Dis 8:289–298. https://doi.org/10.1111/J.1365-2761.1985.TB00945.X
Alexander J, Barregard L, Bignami M, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl-Kraupp B, Hogstrand C, Hoogenboom L, Katrine Knutsen H, Stefano Nebbia C, Oswald I, Petersen A, Maria Rogiers V, Rose M, Roudot A-C, Schwerdtle T, Vleminckx C, Vollmer U, Wallace H (2016) Malachite green in food. EFSA J 14:e04530. https://doi.org/10.2903/J.EFSA.2016.4530
Ali I, Singh P, Aboul-Enein HY, Sharma B (2009) Chiral analysis of ibuprofen residues in water and sediment. Anal Lett 42(12):1747–1760
Ali I, Alharbi OM, ALOthman ZA, Alwarthan A, Al-Mohaimeed AM (2019a) Preparation of a carboxymethylcellulose-iron composite for uptake of atorvastatin in water. Int J Biol Macromol 132:244–253
Ali I, Kucherova A, Memetov N, Pasko T, Ovchinnikov K, Pershin V et al (2019b) Advances in carbon nanomaterials as lubricants modifiers. J Mol Liq 279:251–266
Ali I, Burakova I, Galunin E, Burakov A, Mkrtchyan E, Melezhik A, Kurnosov D, Tkachev A, Grachev V (2019c) High-speed and high-capacity removal of methyl orange and malachite green in water using newly developed mesoporous carbon: kinetic and isotherm studies ACS. Omega 4(21):19293–19306. https://doi.org/10.1021/acsomega.9b02669
Ali I, Burakov AE, Melezhik AV, Babkin AV, Burakova IV, Neskomornaya MEA, Galunin EV, Tkachev AG, Kuznetsov DV (2019d) Removal of copper(II) and zinc(II) ions in water on a newly synthesized polyhydroquinone/graphene nanocomposite material: kinetics, thermodynamics and mechanism. ChemistrySelect 4:12708
Ali I, Afshinb S, Poureshgh Y et al (2020) Green preparation of activated carbon from pomegranate peel coated with zero-valent iron nanoparticles (nZVI) and isotherm and kinetic studies of amoxicillin removal in water. Environ Sci Pollut Res 27:36732–36743. https://doi.org/10.1007/s11356-020-09310-1
Ali I, Babkin AV, Burakova IV, Burakov AE, Neskoromnaya EA, Tkachev AG, Panglisch S, AlMasoud N, Alomar TS (2021a) Fast removal of samarium ions in water on highly efficient nanocomposite-based graphene oxide modified with polyhydroquinone: Isotherms, kinetics, thermodynamics and desorption. J Mol Liq 329:115584. https://doi.org/10.1016/j.molliq.2021.115584. (ISSN 0167-7322)
Ali I, Kon’kova T, Kasianov V, Rysev A, Panglisch S, Mbianda XY et al (2021b) Preparation and characterization of nano-structured modified montmorillonite for dioxidine antibacterial drug removal in water. J Mol Liq 331:115770
Al-Shaalan NH, Ali I, ALOthman ZA, Al-Wahaibi LH, Alabdulmonem H (2019) High performance removal and simulation studies of diuron pesticide in water on MWCNTs. J Mol Liq 289:111039
Altayb HN, Kouidhi B, Baothman OS, Abdulhakim JA, Ayed L, Hager M, Chaieb K (2021) Mathematical modeling and optimization by the application of full factorial design and response surface methodology approach for decolourization of dyes by a newly isolated Photobacterium ganghwense. J Water Proc Eng 2021(44):1–13. https://doi.org/10.1016/j.jwpe.2021.102429
Amin MT, Alazba AA, Shafiq M (2020) LDH of NiZnFe and its composites with carbon nanotubes and data-palm biochar with efficient adsorption capacity for RB5 dye from aqueous solutions: isotherm, kinetic, and thermodynamics studies. Curr Appl Phys. https://doi.org/10.1016/J.CAP.2020.07.005
Badri AF, Siregar PMSBN, Palapa NR, Mohadi R, Mardiyanto M, Lesbani A (2021) Mg-Al/biochar composite with stable structure for malachite green adsorption from aqueous solutions. Bull Chem React Eng Catal 16:149–160. https://doi.org/10.9767/BCREC.16.1.10270.149-160
Balasubramanyan S, Arayathody S, Sugunan S, Narayanan BN (2018) Selective liquid phase oxidation of cyclohexene over magnetic Fe3O4/graphene oxide nanocomposite. Mater Chem Phys 211:23–33. https://doi.org/10.1016/J.MATCHEMPHYS.2018.02.006
Basheer AA (2018) New generation nano-adsorbents for the removal of emerging contaminants in water. J Mol Liq. https://doi.org/10.1016/j.molliq.2018.04.021. (ISSN 0167-7322)
Basheer AA (2020) Advances in the smart materials applications in the aerospace industries. Aircr Eng Aerosp Technol 92(7):1027–1035
Bautin VA, Seferyan AG, Nesmeyanov MS, Usov NA (2017) Magnetic properties of polycrystalline cobalt nanoparticles. AIP Adv 7:045103. https://doi.org/10.1063/1.4979889
Biata NR, Jakavula S, Mashile GP, Nqombolo A, Moutloali RM, Nomngongo PN (2020) Recovery of gold(III) and iridium(IV) using magnetic layered double hydroxide (Fe3O4/Mg-Al-LDH) nanocomposite: Equilibrium studies and application to real samples. Hydrometallurgy 197:105447. https://doi.org/10.1016/J.HYDROMET.2020.105447
Borges GA, Ferreira GMD, Siqueira KPF, Dias A, Navarro KON, Silva SJBE, Rodrigues GD, Mageste AB (2020) Adsorption of organic and inorganic arsenic from aqueous solutions using MgAl-LDH with incorporated nitroprusside. J Colloid Interface Sci 575:194–205. https://doi.org/10.1016/J.JCIS.2020.04.078
Burakova IV, Burakov AE, Tkachev AG, Troshkina ID, Veselova OA, Babkin AV, Aung WM, Ali I (2017) Kinetics of the adsorption of scandium and cerium ions in sulfuric acid solutions on a nanomodified activated carbon. The address for the corresponding author was captured as affiliation for all authors. Please check if appropriate. Molliq. https://doi.org/10.1016/j.molliq.2018.01.063
Chen T, Quan X, Ji Z, Li X, Pei Y (2020) Synthesis and characterization of a novel magnetic calcium-rich nanocomposite and its remediation behaviour for As(III) and Pb(II) co-contamination in aqueous systems. Sci Total Environ 706:135122. https://doi.org/10.1016/J.SCITOTENV.2019.135122
Chilukoti S, Thangavel T (2019) Enhanced adsorption of Congo red on microwave synthesized layered Zn-Al double hydroxides and its adsorption behaviour using mixture of dyes from aqueous solution. Inorg Chem Commun 100:107–117. https://doi.org/10.1016/J.INOCHE.2018.12.027
Cui H, Liu Y, Ren W (2013) Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature sol–gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv Powder Technol 24:93–97. https://doi.org/10.1016/J.APT.2012.03.001
da Silva Santos DH, Paulino JCPL, dos Santos Alves GF et al (2021) Effluent treatment using activated carbon adsorbents: a bibliometric analysis of recent literature. Environ Sci Pollut Res 28:32224–32235. https://doi.org/10.1007/s11356-021-14267-w
da Silva AF, da Silva Duarte JL, Selvasembian R et al (2023) Life cycle assessment of LDH-MgFe production for nitrate removal: impacts of synthesis methods. J Nanopart Res 25:16. https://doi.org/10.1007/s11051-022-05662-6
De Oliveira FF, Moura KO, Costa LS, Vidal CB, Loiola AR, Do Nascimento RF (2020) Reactive adsorption of parabens on synthesized micro- and mesoporous silica from coal fly ash: PH effect on the modification process. ACS Omega 5:3346–3357. https://doi.org/10.1021/ACSOMEGA.9B03537/ASSET/IMAGES/LARGE/AO9B03537_0004.JPEG
De Roy A, Forano C, Besse JP (2006) Layered double hydroxides: present and future. In: Rives V (ed). edn. Nova Science Publishers, Clermont-Fd, p. 1–39
dos Santos GE, dos Santos Lins PV, de Magalhães Oliveira LM, da Silva EO, Anastopoulos I, Erto A, Giannakoudakis DA, de Almeida AR, da Silva Duarte JL, Meili L (2021) Layered double hydroxides/biochar composites as adsorbents for water remediation applications: recent trends and perspectives. J Clean Prod 284:124755. https://doi.org/10.1016/J.JCLEPRO.2020.124755
Dou L, Zhang H (2016) Facile assembly of nanosheet array-like CuMgAl-layered double hydroxide/rGO nanohybrids for highly efficient reduction of 4-nitrophenol. J Mater Chem A 4:18990–19002. https://doi.org/10.1039/C6TA08313G
Drici-Setti N, Lelli P, Jouini N (2020) LDH-Co-Fe-acetate: a new efficient sorbent for azoic dye removal and elaboration by hydrolysis in polyol, characterization, adsorption, and anionic exchange of direct red 2 as a model anionic dye. Material (basel, Switzerland). https://doi.org/10.3390/MA13143183
Duarte FdS, Melo ALMdS, Ferro AdB, Zanta CLdPeS, Duarte JLdS, Oliveira RMPB (2022) Magnetic zinc oxide/manganese ferrite composite for photodegradation of the antibiotic rifampicin. Materials 15:8185. https://doi.org/10.3390/ma15228185
Dutt MA, Hanif MA, Nadeem F, Bhatti HN (2020) A review of advances in engineered composite materials popular for wastewater treatment. J Environ Chem Eng 8:104073. https://doi.org/10.1016/J.JECE.2020.104073
El-Dib FI, Mohamed DE, El-Shamy OAA, Mishrif MR (2020) Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt J Pet 29:1–7. https://doi.org/10.1016/J.EJPE.2019.08.004
Everaert M, Degryse F, McLaughlin MJ, De Vos D, Smolders E (2017) Agronomic effectiveness of granulated and powdered P-exchanged Mg-Al LDH relative to struvite and MAP. J Agric Food Chem 65:6736–6744. https://doi.org/10.1021/ACS.JAFC.7B01031/SUPPL_FILE/JF7B01031_SI_001.PDF
Freundlich HM (1906) Over the adsorption in solution. J Phys Chem A 57:385–470
Frost RL, Scholz R, López A, Theiss FL (2014) Vibrational spectroscopic study of the natural layered double hydroxide manasseite now defined as hydrotalcite-2H—Mg6Al2(OH)16[CO3]⋅4H2O. Spectrochim Acta Part A Mol Biomol Spectrosc 118:187–191. https://doi.org/10.1016/J.SAA.2013.08.105
George G, Saravanakumar MP (2018) Facile synthesis of carbon-coated layered double hydroxide and its comparative characterisation with Zn–Al LDH: application on crystal violet and malachite green dye adsorption—isotherm, kinetics and Box-Behnken design. Environ Sci Pollut Res 2018(25):30236–30254. https://doi.org/10.1007/S11356-018-3001-3
GhiasiMoaser A, Khoshnavazi R (2017) Facile synthesis and characterization of Fe3O4@MgAl-LDH@STPOM nanocomposites for highly enhanced and selective degradation of methylene blue. New J Chem 41:9472–9481. https://doi.org/10.1039/C7NJ00792B
Guo J, Shi L, Wu L, Suharyadi E, Hermawan A, Puspitarum DL (2018) Crystal structure and magnetic properties of magnesium ferrite (MgFe2O4) nanoparticles synthesized by coprecipitation method. J Phys Conf Ser 1091:012003. https://doi.org/10.1088/1742-6596/1091/1/012003
Hedayatnasab Z, Abnisa F, Daud WMAW (2017) Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater Des 123:174–196. https://doi.org/10.1016/J.MATDES.2017.03.036
Henrique DC, Quitela DU, Ide AH, Lins PVS, Perazzini MTB, Perazzini H, Oliveira LMTM, Duarte JLS, Meili L (2021) Mollusk shells as adsorbent for removal of endocrine disruptor in different water matrix. J Environ Chem Eng 9:105704. https://doi.org/10.1016/J.JECE.2021.105704
Henrique DC, Henrique DC, Solano JRS, Barbosa VT, Silva AOS, Dornelas CB, Duarte JLS, Meili L (2022) Calcined Mytella falcata shells as a source for CaAl/LDH production: synthesis and characterization. Colloids Surf A Physicochem Eng Aspects 644:128752 (ISSN 0927-7757)
Ho YS, McKay G (1998) A Comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot. https://doi.org/10.1205/095758298529696
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Jeyagowri B, Yamuna RT (2015) Potential efficacy of a mesoporous biosorbent Simarouba glauca seed shell powder for the removal of malachite green from aqueous solutions. New Pub Balaban 57:11326–11336. https://doi.org/10.1080/19443994.2015.1042060
Jiang LL, Yu HT, Pei LF, Hou XG (2018) The effect of temperatures on the synergistic effect between a magnetic field and functionalized graphene oxide-carbon nanotube composite for Pb2+ and phenol adsorption. J Nanomater. https://doi.org/10.1155/2018/9167938
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. https://doi.org/10.1021/ja02242a004
Lins PVS, Henrique DC, Ide AH, Dotto GL, Yazidi A, Sellaoui L, Erto A, Meili L (2020) Adsorption of a non-steroidal anti-inflammatory drug onto MgAl/LDH-activated carbon composite–experimental investigation and statistical physics modeling. Colloids Surf A Physicochem Eng Aspects 586:124217
Lu L, Li J, Ng DHL, Yang P, Song P, Zuo M (2017) Synthesis of novel hierarchically porous Fe3O4@MgAl–LDH magnetic microspheres and its superb adsorption properties of dye from water. J Ind Eng Chem 46:315–323. https://doi.org/10.1016/J.JIEC.2016.10.045
Lu C, Kim TH, Bendix J, Abdelmoula M, Ruby C, Nielsen UG, Bruun Hansen HC (2020) Stability of magnetic LDH composites used for phosphate recovery. J Colloid Interface Sci 580:660–668. https://doi.org/10.1016/J.JCIS.2020.07.020
Mansour SF, Al-Wafi R, Ahmed MK, Wageh S (2020) Microstructural, morphological behavior and removal of Cr(VI) and Se(IV) from aqueous solutions by magnetite nanoparticles/PVA and cellulose acetate nanofibers. Appl Phys A Mater Sci Process 126:1–14. https://doi.org/10.1007/S00339-020-3377-Z/FIGURES/10
Meili L, Lins PV, Zanta CLPS, Soletti JI, Ribeiro LMO, Dornelas CB, Silva TL, Vieira MGA (2019) MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl Clay Sci 168:11–20. https://doi.org/10.1016/J.CLAY.2018.10.012
Melo JMO, Duarte JLS, Ferro AB, Meili L, Zanta CLPS (2020) Comparing electrochemical and fenton-based processes for aquaculture biocide degradation. Water Air Soil Pollut 231:1–14. https://doi.org/10.1007/S11270-020-4454-9/TABLES/5
Milonjić SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serbian Chem Soc. https://doi.org/10.2298/JSC0712363M
Morimoto K, Tamura K, Iyi N, Ye J, Yamada H (2011) Adsorption and photodegradation properties of anionic dyes by layered double hydroxides. J Phys Chem Solids 72:1037–1045. https://doi.org/10.1016/J.JPCS.2011.05.018
Nikić J, Watson MA, Isakovski MK, Tubić A, Šolić M, Kordić B, Agbaba J (2021) Synthesis, characterization and application of magnetic nanoparticles modified with Fe-Mn binary oxide for enhanced removal of As(III) and As(V). Environ Technol 42:2527–2539. https://doi.org/10.1080/09593330.2019.1705919
Palapa NR, Taher T, Mohadi R, Said M, Lesbani A (2018) Synthesis of Ni/Al layered double hydroxides (LDHs) for adsorption of malachite green and direct yellow dyes from solutions: Kinetic and thermodynamic. AIP Conf Proc 2026:020033. https://doi.org/10.1063/1.5064993
Palapa NR, Mohadi R, Rachmat A, Lesbani A (2020) Adsorption study of malachite green removal from aqueous solution using Cu/M3+ (M3+=Al, Cr) layered double hydroxide. Mediterr J Chem 10:33–45. https://doi.org/10.13171/MJC10102001261236AL
Pérez-Ramírez J, Mul G, Kapteijn F, Moulijn JA (2001) In situ investigation of thethermal decomposition of Co–Al hydrotalcite in different atmospheres. J Mater Chem 11:821–830. https://doi.org/10.1039/B009320N
Perveen S, Nadeem R, Iqbal M, Bibi S, Gill R, Saeed R, Noreen S, Akhtar K, MehmoodAnsari T, Alfryyan N (2021) Graphene oxide and Fe3O4 composite synthesis, characterization and adsorption efficiency evaluation for NO3¯ and PO43¯ ions in aqueous medium. J Mol Liq 339:116746. https://doi.org/10.1016/J.MOLLIQ.2021.116746
Quintela DU, Henrique DC, dos Santos Lins PV, Ide AH, Erto A, da Duarte JLS, Meili L (2020) Waste of Mytella Falcata shells for removal of a triarylmethane biocide from water: kinetic, equilibrium, regeneration and thermodynamic studies. Colloids Surfaces B Biointerfaces 195:111230. https://doi.org/10.1016/J.COLSURFB.2020.111230
Qureshi UA, Hameed BH, Ahmed MJ (2020) Adsorption of endocrine disrupting compounds and other emerging contaminants using lignocellulosic biomass-derived porous carbons: a review. J Water Process Eng. 38:101380. https://doi.org/10.1016/J.JWPE.2020.101380
Rahimi Z, Sarafraz H, Alahyarizadeh G, Shirani AS (2018) Hydrothermal synthesis of magnetic CoFe2O4 nanoparticles and CoFe2O4/MWCNTs nanocomposites for U and Pb removal from aqueous solutions. J Radioanal Nucl Chem 317:431–442. https://doi.org/10.1007/S10967-018-5894-1/TABLES/4
Rajabi M, Mahanpoor K, Moradi O (2019) Preparation of PMMA/GO and PMMA/GO-Fe3O4 nanocomposites for malachite green dye adsorption: Kinetic and thermodynamic studies. Compos Part B Eng 167:544–555. https://doi.org/10.1016/J.COMPOSITESB.2019.03.030
Rasheed T, Bilal M, Nabeel F, Adeel M, Iqbal HMN (2019) Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ Int 122:52–66. https://doi.org/10.1016/J.ENVINT.2018.11.038
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem. https://doi.org/10.1021/j150576a611
Rizal C, Kolthammer J, Pokharel RK, Choi BC (2013) Magnetic properties of nanostructured Fe-Co alloys. J Appl Phys 113:113905. https://doi.org/10.1063/1.4795267
Rodriguez-Narvaez OM, Peralta-Hernandez JM, Goonetilleke A, Bandala ER (2017) Treatment technologies for emerging contaminants in water: a review. Chem Eng J 323:361–380. https://doi.org/10.1016/J.CEJ.2017.04.106
Saju D, Sabu T (2020) Layered double hydroxides: fundamentals to applications. Layer Double Hydroxide Polym Nanocomposites. https://doi.org/10.1016/B978-0-08-101903-0.00001-5
Sepúlveda P, Erto A, da Duarte JLS, Meili L (2021) Fundamentals of adsorption in liquid phase. Springer International Publishing, Cham, pp 1–24. https://doi.org/10.1007/978-3-030-64092-7_1
Shan RR, Yan LG, Yang YM, Yang K, Yu SJ, Yu HQ, Zhu BC, Du B (2015) Highly efficient removal of three red dyes by adsorption onto Mg–Al-layered double hydroxide. J Ind Eng Chem 21:561–568. https://doi.org/10.1016/J.JIEC.2014.03.019
Shen J, Zhou Y, Li S, Gu P, Xue G (2019) Hydrogel-coated Fe3O4 nanoparticles as an efficient heterogeneous Fenton catalyst for degradation of phenol. J Mater Sci 54:10684–10694. https://doi.org/10.1007/S10853-019-03661-Y/FIGURES/9
Shimizu S, Matubayasi N (2021) Sorption: a statistical thermodynamic fluctuation theory. Langmuir 37:7380–7391. https://doi.org/10.1021/ACS.LANGMUIR.1C00742
Shin S, Yoon H, Jang J (2008) Polymer-encapsulated iron oxide nanoparticles as highly efficient Fenton catalysts. Catal Commun 10:178–182
Shokrollahi H (2017) A review of the magnetic properties, synthesis methods and applications of maghemite. J Magn Magn Mater 426:74–81. https://doi.org/10.1016/J.JMMM.2016.11.033
Silveira JE, de Souza AS, Pansini FN, Ribeiro AR, Scopel WL, Zazo J, A.,et al. (2023) A comprehensive study of the reduction of nitrate on natural FeTiO3: Photocatalysis and DFT calculations. Separation Purif Technol 306:122570
Sips R (1948) On the structure of a catalyst surface. J Chem Phys Doi 10(1063/1):1746922
Stoia M, Istratie R, Păcurariu C (2016) Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J Therm Anal Calorim 125:1185–1198. https://doi.org/10.1007/S10973-016-5393-Y/FIGURES/14
Sun L, Hu S, Sun H, Guo H, Zhu H, Liu M, Sun H (2015) Malachite green adsorption onto Fe3O4@SiO2-NH2: isotherms, kinetic and process optimization. RSC Adv 5:11837–11844. https://doi.org/10.1039/C4RA13402H
Tang S, Yao Y, Chen T, Kong D, Shen W, Lee HK (2020) Recent advances in the application of layered double hydroxides in analytical chemistry: a review. Anal Chim Acta 1103:32–48. https://doi.org/10.1016/J.ACA.2019.12.065
Tavares MG, da Duarte JLS, Oliveira LMTM, Fonseca EJS, Tonholo J, Ribeiro AS, Zanta CLPS (2022) Reusable iron magnetic catalyst for organic pollutant removal by adsorption, Fenton and photo Fenton process. J Photochem Photobiol A Chem 432:114089. https://doi.org/10.1016/J.JPHOTOCHEM.2022.114089
Tirtom VN, Dinçer A, Becerik S, Aydemir T, Çelik A (2012) Desalination and water treatment removal of lead (II) ions from aqueous solution by using crosslinked chitosan-clay beads. Desalination Water Treat 39:76–82
Wang J, Guo X (2020) Adsorption isotherm models: classification, physical meaning, application and solving method. Chemosphere 258:127279. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127279
Xing Y, Bai XH, Peng ML, Ma XR, Buske N, Cui YL (2019) Recyclable Fe3O4/Au nanocomposites for oxidation degradation of methylene blue in near neutral solution. NANO 14:1950122. https://doi.org/10.1142/S1793292019501224
Yang Q, Lv K, Liang L, Ma J, Liu C, Yan X, Wang M, Yao H, Wei D, Ma D, Xie K (2021) Layered double hydroxides as high-performance anode material for potassium ion battery. J Alloys Compd 882:160711. https://doi.org/10.1016/J.JALLCOM.2021.160711
Yuan ML, Tao JH, Yu L, Song C, Qiu GZ, Li Y, Xu ZH (2011) Synthesis and magnetic properties of Fe–Ni Alloy nanoparticles obtained by hydrothermal reaction. Adv Mater Res 239–242:748–753. https://doi.org/10.4028/WWW.SCIENTIFIC.NET/AMR.239-242.748
Yue X, Liu W, Chen Z, Lin Z (2017) Simultaneous removal of Cu(II) and Cr(VI) by Mg–Al–Cl layered double hydroxide and mechanism insight. J Environ Sci 53:16–26. https://doi.org/10.1016/J.JES.2016.01.015
Zhang YX, Hao XD, Kuang M, Zhao H, Wen ZQ (2013) Preparation, characterization and dye adsorption of Au nanoparticles/ZnAl layered double oxides nanocomposites. Appl Surf Sci 283:505–512. https://doi.org/10.1016/J.APSUSC.2013.06.136
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) and Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL/Brazil). José Leandro da Silva Duarte thank (CAPES/Brazil) for the Grant (88882.316136/2019-01).
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) and Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL/Brazil). José Leandro da Silva Duarte thank (CAPES/Brazil) for the grant (88882.316136/2019-01).
Author information
Authors and Affiliations
Contributions
ÍMGLS: conceptualization, methodology, validation, formal analysis, investigation, data curation. IMA: investigation. KJLS: data curation. LFAMO: formal analysis, data curation. EJSF: formal analysis, data curation. LMTMO: formal analysis, resources, writing—review and editing. CLPSZ: conceptualization, methodology, resources, writing—review and editing, visualization, supervision, project administration, funding acquisition. JLSD: conceptualization, methodology, resources, writing—review and editing, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Sá, Í.M.G.L., Agra, I.d., Leite, K.J.d. et al. Magnetic MgAl-LDH for Adsorptive Removal of Malachite Green from Water. Int J Environ Res 17, 30 (2023). https://doi.org/10.1007/s41742-023-00521-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-023-00521-1