Abstract
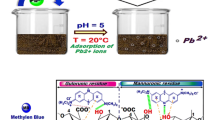
Environmental friendly activated carbon with microporous structures have been synthesized by surface modification procedure using concentrated H2SO4. Various factors affecting the adsorption properties of methylene blue (MB) dye onto algae—raw Enteromorpha intestinalis (REI) and sulphuric acid modified E. intestinalis (SMEI)—have been discussed, such as time, pH, biosorbent dose, MB dye concentration, and temperature. FTIR spectroscopy, SEM, and TGA analysis investigated the newly synthesized biosorbent. The relationship between the structure and adsorption properties of E. intestinalis had been discussed based on the experimental and theoretical data. Langmuir monolayer adsorption capacity of the adsorbent—REI and SMEI—was calculated as 76.28 and 95.27 mg/g, respectively. The batch data followed Freundlich isotherm model amongst four isotherms and pseudo-second-order rate equation. The results exhibit that adsorption of MB dye onto REI and SMEI was multilayer adsorption. Thermodynamic parameters revealed spontaneity of adsorption and the nature was chemisorption. Inferable from attributes of minimal effort, high-effectiveness, and vitality sparing, E. intestinalis can be utilized as a productive adsorbent for the removal of dyes from industrial wastewater.
Graphical Abstract

Article Highlights
-
A novel modified algae material—E. intestinalis—was prepared for the adsorption of MB dye.
-
The optimum adsorption conditions of E. intestinalis towards MB dye are investigated.
-
Adsorption behaviour follows Freundlich isotherm and pseudo-second order kinetic model.
-
Thermodynamic study demonstrates that the present study is spontaneous, exothermic, and physical.










Similar content being viewed by others
References
AL-Othman ZA, Inamuddin, Naushad M (2011) Adsorption thermodynamics of trichloroacetic acid herbicide on polypyrrole Th(IV) phosphate composite cation-exchanger. Chem Eng J 169:38–42
Asghar A, Raman AAA, Daud WMAW (2015) Advanced oxidation processes for in situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J Clean Prod 87:826–838
Bilal M, Asgher M, Shahid M, Bhatti HN (2016) Characteristic features and dye degrading capability of agar–agar gel immobilized manganese peroxidise. Int J Biol Macromol 86:728–740
Chavaco LC, Arcos CA, Prato-Garcia D (2017) Decolorization of reactive dyes in solar pond reactors: perspectives and challenges for the textile industry. J Environ Manag 198:203–212
Chen X, Deng Q, Lin S, Du C, Zhao S, Hu Y, Yang Z, Lyu Y, Han J (2017) A new approach for risk assessment of aggregate dermal exposure to banned azo dyes in textiles. Regul Toxicol Pharmacol 91:173–178
Chiong T, Lau SY, Lek ZH, Boon YK, Danquah MK (2016) Enzymatic treatment of methyl orange dye in synthetic wastewater by plant-based peroxidase enzymes. J Environ Chem Eng 4:2500–2509
Dotto GL, Pinto LA (2013) Adsorption of food dyes onto chitosan: optimization process and kinetic. Carbohydr Polym 84:231–238
Duan XH, Srinivasakannan C, Wang X, Wang F, Liu XY (2017) Synthesis of activated carbon fibers from cotton by microwave induced H3PO4 activation. J Taiwan Inst Chem 70:374–381
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. Chem Zentralblatt 1:875–890
Fajardo AS, Martins RC, Silva DR, Martinez-Huitle CA, Quinta-Ferreira RM (2017) Dye wastewaters treatment using batch and recirculation flow electrocoagulation systems. J Electroanal Chem 801:30–37
Fatima M, Farooq R, Lindstrom RW, Saeed M (2017) A review on biocatalytic decomposition of azo dyes and electrons recovery. J Mol Liq 246:275–281
Feng Z, Yu J, Kong J, Wang T (2016) A novel porous Al2O3 layer/AgNPs–Hemin composite for degradation of azo dyes under visible and UV irradiation. Chem Eng J 294:236–245
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Gil A, Assis FCC, Albeniz S, Korili SA (2011) Removal of dyes from wastewaters by adsorption on pillared clays. Chem Eng J 168:1032–1040
Guo J, Zhang Q, Cai Z, Zhao K (2016) Preparation and dye filtration property of electrospun polyhydroxybutyrate–calcium alginate/carbon nanotubes composite nanofibrous filtration membrane. Sep Purif Technol 161:69–79
Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat MA (2013) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv Colloid Interface Sci 193:24–31
Gurses A, Dogar C, Yalcin M, Acikyildiz M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye methylene blue onto clay. J Hazard Mater 131:217–228
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Jadhav AJ, Holkar CR, Karekar SE (2015) Ultrasound assisted manufacturing of paraffin wax nanoemulsions: process optimization. Ultrason Sonochem 23:201–207
Jothirani R, Kumar PS, Saravanan A, Narayan AS, Dutta A (2016) Ultrasonic modified corn pith for the sequestration of dye from aqueous solution. J Ind Eng Chem 39:162–175
Kaith BS, Dhiman J, Bhatia JK (2015) Preparation and application of grafted Holarrhena antidycentrica fiber as cation exchanger for adsorption of dye from aqueous solution. J Environ Chem Eng 3:1038–1046
Kausar A, Iqbal M, Javed A, Aftab K, Nazli Z, Bhatti HN, Nouren S (2018) Dyes adsorption using clay and modified clay: a review. J Mol Liq 256:395–407
Kono H, Kusumoto R (2015) Removal of anionic dyes in aqueous solution by flocculation with cellulose ampholytes. J Water Process Eng 7:83–93
Kumar PS, Ramalingam S, Sathishkumar K (2011) Removal of methylene blue dye from aqueous solution by activated carbon prepared from cashew nut shell as a new low-cost adsorbent. Korean J Chem Eng 28:149–155
Kumar PS, Fernando PSA, Ahmed RT, Srinath R, Priyadharshini M, Vignesh AM, Thanjiappan A (2014a) Effect of temperature on the adsorption of methylene blue dye onto sulfuric acid–treated orange peel. Chem Eng Commun 201:1526–1547
Kumar PS, Sivaranjanee R, Vinothini U, Raghavi M, Rajasekar K, Ramakrishnan K (2014b) Adsorption of dye onto raw and surface modified tamarind seeds: isotherms, process design, kinetics and mechanism. Desalin Water Treat 52:2620–2633
Kumar PS, Palaniyappan M, Priyadharshini M, Vignesh AM, Thanjiappan A, Fernando PSA, Ahmed RT, Srinath R (2014c) Adsorption of basic dye onto raw and surface-modified agricultural waste. Environ Prog Sustain Energy 33:87–98
Kumar PS, Varjani SJ, Suganya S (2018) Treatment of dye wastewater using an ultrasonic aided nanoparticle stacked activated carbon: kinetic and isotherm modelling. Bioresour Technol 250:716–722
Lagergren S (1898) About the theory of so-called adsorption of soluble substance. Kungliga Sven Vetenskapsakademiens Handl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lonappan L, Rouissi T, Das RK, Brar SK, Ramirez AA, Verma M, Surampalli RY, Valero JR (2016) Adsorption of methylene blue on biochar microparticles derived from different waste materials. Waste Manag 49:537–544
Low MJD (1960) Kinetics of chemisorption of gases on solids. Chem Rev 60:267–312
Manikandan G, Kumar PS, Saravanan A (2018) Modelling and analysis on the removal of methylene blue dye from aqueous solution using physically/chemically modified Ceiba pentandra seeds. J Ind Eng Chem 62:446–461
Miyah Y, Lahrichi A, Idrissi M, Khalil A, Zerrouq F (2018) Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: equilibrium and kinetic studies. Surf Interfaces 11:74–81
Mouni L, Belkhiri L, Bollinger J-C, Bouzaza A, Assadi A, Tirri A, Dahmoune F, Madani K, Remini H (2018) Removal of methylene blue from aqueous solutions by adsorption on kaolin: kinetic and equilibrium studies. Appl Clay Sci 153:38–45
Munagapati VS, Kim D (2016) Adsorption of anionic azo dye congo red from aqueous solution by cationic modified orange peel powder. J Mol Liq 220:540–548
Nakata H, Uehara K, Goto Y, Fukumura M, Shimasaki H, Takikawa K (2014) Polycyclic aromatic hydrocarbons in oysters and sediments from the Yatsushiro Sea, Japan: comparison of potential risks among PAHs, dioxins and dioxin-like compounds in benthic organisms. Ecotoxicol Environ Saf 99:61–68
Pang J, Fu F, Ding Z, Lu J, Li N, Tang B (2017) Adsorption behaviors of methylene blue from aqueous solution on mesoporous birnessite. J Taiwan Inst Chem Eng 77:168–176
Salleh MAM, Mahmoud DK, Karim WAWA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280:1–13
Sham AYW, Notley SM (2018) Adsorption of organic dyes from aqueous solutions using surfactant exfoliated graphene. J Environ Chem Eng 6:495–504
Shuhong Y, Meiping Z, Hong Y, Han W, Shan X, Yan L, Jihiu W (2014) Biosorption of Cu(2+), Pb(2+) and Cr(6+) by a novel exopolysaccharide from Arthrobacter ps-5. Carbohydr Polym 101:50–56
Suganya S, Kumar PS, Saravanan A, Rajan PS, Ravikumar C (2017) Computation of adsorption parameters for the removal of dye from wastewater by microwave assisted sawdust: theoretical and experimental analysis. Environ Toxicol Pharmacol 50:45–57
Sun C, Zhang J, Ma Q, Chen Y (2015) Human health and ecological risk assessment of polycyclic aromatic hydrocarbons in drinking source water from a large mixed-use reservoir. Int J Environ Res Public Health 12:13956–13969
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physicochim URSS 12:217–225
Tharaneedhar V, Kumar PS, Saravanan A, Ravikumar C, Jaikumar V (2017) Prediction and interpretation of adsorption parameters for the sequestration of methylene blue dye from aqueous solution using microwave assisted corncob activated carbon. Sustain Mater Technol 11:1–11
Tong DS, Wu CW, Adebajo MO, Jin GC, Yu WH, Ji SF, Zhou CH (2018) Adsorption of methylene blue from aqueous solution onto porous cellulose-derived carbon/montmorillonite nanocomposites. Appl Clay Sci 161:256–264
Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA (2013) Graphite oxide/chitosan composite for reactive dye removal. Chem Eng J 217:256–265
Wan CC, Jiao Y, Qiang TG, Li J (2017) Cellulose-derived carbon aerogels supported goethite (–FeOOH) nanoneedles and nanoflowers for electromagnetic interference shielding. Carbohydr Polym 156:427–434
Wang S, Zhu ZH (2006) Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution. J Hazard Mater 136:946–952
Xiao H, Zhao T, Li C-H, Li M-Y (2017) Eco-friendly approaches for dyeing multiple type of fabrics with cationic reactive dyes. J Clean Prod 165:1499–1507
Zahrim AY, Hilal N (2013) Treatment of highly concentrated dye solution by coagulation/flocculation–sand filtration and nanofiltration. Water Resour Ind 3:23–34
Zhai X, Xia J, Zhang Y (2017) Integrated approach of hydrological and water quality dynamic simulation for anthropogenic disturbance assessment in the Huai River Basin, China. Sci Total Environ 598:749–764
Zhang S, Wang Z, Zhang Y, Pan H, Tao L (2016) Adsorption of methylene blue on organosolv lignin from rice straw. Procedia Environ Sci 31:3–11
Zhang J, Li F, Sun Q (2018) Rapid and selective adsorption of cationic dyes by a unique metal–organic framework with decorated pore surface. Appl Surf Sci 440:1219–1226
Zhu M-X, Lee L, Wang H-H, Wang Z (2007) Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J Hazard Mater 149:735–741
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest for this research article.
Rights and permissions
About this article
Cite this article
Saravanan, A., Kumar, P.S., Yaashikaa, P.R. et al. Modelling on the Removal of Dye from Industrial Wastewater Using Surface Improved Enteromorpha intestinalis. Int J Environ Res 13, 349–366 (2019). https://doi.org/10.1007/s41742-019-00181-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-019-00181-0