Abstract
Background and Objective
Hyperkalaemia can be a life-threatening condition, particularly in patients with advanced chronic kidney disease with and without heart failure. Renin-angiotensin-aldosterone system inhibitor therapy offers cardiorenal protection in chronic kidney disease and heart failure; however, it may also cause hyperkalaemia subsequently resulting in down-titration or discontinuation of treatment. Hence, there is an unmet need for hyperkalaemia treatment in patients with chronic kidney disease with and without heart failure to enable renin-angiotensin-aldosterone system inhibitor use in this patient population. In this study, we develop a de novo disease progression and cost-effectiveness model to evaluate the clinical and economic outcomes associated with the use of patiromer for the treatment of hyperkalaemia in patients with chronic kidney disease with and without heart failure.
Methods
A Markov model was developed using data from the OPAL-HK trial to assess the health economic impact of patiromer therapy in comparison to standard of care in controlling hyperkalaemia in patients with advanced chronic kidney disease with and without heart failure in the Irish setting. The model was designed to predict the natural history of chronic kidney disease and heart failure and quantify the costs and benefits associated with the use of patiromer for hyperkalaemia management over a lifetime horizon from a payer perspective.
Results
Treatment with patiromer was associated with an increase in discounted life-years (8.62 vs 8.37) and an increase in discounted quality-adjusted life-years (6.15 vs 5.95). Incremental discounted costs were predicted at €4979 per patient, with an incremental cost-effectiveness ratio of €25,719 per quality-adjusted life-year gained. Patients remained taking patiromer treatment for an average of 7.7 months, with treatment associated with reductions in the overall clinical event incidence and a delay in chronic kidney disease progression. Furthermore, patiromer was associated with lower overall rates of hospitalisation, major adverse cardiovascular events, dialysis, renin-angiotensin-aldosterone system inhibitor discontinuation episodes and renin-angiotensin-aldosterone system inhibitor down-titration episodes. At a willingness-to-pay threshold of €45,000 per quality-adjusted life-year in Ireland, treatment with patiromer was estimated to have a 100% chance of cost effectiveness compared with standard of care.
Conclusions
This study has demonstrated an economic case for the reimbursement of patiromer for the treatment of hyperkalaemia in patients with chronic kidney disease with and without heart failure in Ireland. Patiromer was estimated to improve life expectancy and quality-adjusted life expectancy, whilst incurring marginal additional costs when compared with current standard of care. Results are predominantly attributed to the ability of patiromer to enable the continuation of renin-angiotensin-aldosterone system inhibitor treatment whilst also reducing potassium levels.
Similar content being viewed by others
Clinical trials have demonstrated that patiromer is a safe and effective treatment in reducing hyperkalaemia in patients with chronic kidney disease with and without heart failure. |
This study models the cost effectiveness of patiromer in the Irish healthcare setting and demonstrates clinical and economic benefits associated with the use of patiromer in patients with chronic kidney disease with and without heart failure. |
Decision makers may consider patiromer as a cost-effective treatment in Ireland, with a willingness-to-pay threshold of €45,000 per quality-adjusted life-year. |
1 Introduction
Hyperkalaemia (HK) commonly presents in the clinic and has the potential to be life threatening [1]. It is most prevalent among patients with chronic kidney disease (CKD) and heart failure (HF), particularly those receiving renin angiotensin aldosterone system inhibitor (RAASi) treatment and is a burden on both clinical and economic outcomes [2,3,4,5,6,7,8,9,10].
Patients with HK are at a significantly higher risk of major adverse cardiovascular events (MACE), mortality, hospitalisation and increased healthcare resource utilisation [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. A real-world evidence study investigated the burden of HK in patients with HF [26]. This Danish-based study found that HK and recurrent episodes were significantly high in this population, with four in ten patients with HF developing HK. Furthermore, HK was associated with renal dysfunction and increased occurrence in cardiovascular outcomes, including ventricular arrhythmia, HF readmission and any cardiac diagnosis. A study by Núñez et al. examined the risk of mortality during long-term potassium monitoring in patients with HF [24]. Findings from this study demonstrated that HK was independently associated with mortality, and potassium normalisation significantly reduced the risk of mortality. A retrospective study examined the cost of HK in patients with CKD and/or HF in the USA [11]. Hyperkalaemia was associated with increased total healthcare costs, higher healthcare resource utilisation rates including inpatient admissions, outpatient visits and emergency department visits, greater rehospitalisations and longer hospital length of stay, although these do not necessarily imply causation. Hence, the need for better treatment options in HK management.
Currently, there is a relative paucity in economic evaluations of HK therapies, owing, in part, to the limited treatment options available. Current treatment strategies for the management of HK are suboptimal as they often involve down-titration or discontinuation of RAASi therapy, resulting in increased mortality and disease progression in patients with CKD with and without HF [29,30,31,32,33]. Hence, this strategy of HK management may lead to increased healthcare costs from renal replacement therapy (RRT) and/or cardiovascular hospitalisations. More recently, advances in new treatment options for patients with HK with cardiorenal disease have emerged for the long-term management of HK.
Patiromer, a non-absorbed polymer, binds to potassium within the gastrointestinal tract increasing its faecal excretion. The effectiveness and safety of patiromer up to 1 year have been demonstrated in cardiorenal patients receiving RAASi therapy in phase II and III clinical trials [34,35,36]. Patiromer has also been shown to enable initiation and up-titration of RAASi in patients at risk of HK [37, 38]. Patiromer has already been approved in the USA and European Union and has more recently been recommended by the UK (National Institute for Health and Care Excellence) for treating HK in patients with CKD [39,40,41].
In 2019, the Irish National Centre for Pharmacoeconomics assessment of patiromer was made; however, patiromer has not yet been reimbursed in Ireland. The assessment of the economic value of introducing patiromer in Ireland for the management of HK in patients with CKD, however, has not previously been published, and is needed to help inform decision makers. Therefore, the objective of this study was to develop a de novo decision analytic model capable of modelling the clinical and economic outcomes associated with the use of patiromer for the treatment of HK in patients with CKD with and without HF. A further objective of the study is to evaluate the cost effectiveness of patiromer in the Irish healthcare setting.
2 Methods
2.1 Patiromer OPAL-HK Trial
The modelling approach was developed in order to extrapolate results from the OPAL-HK trial [42]. This trial was used to assess the efficacy and safety of patiromer and was an international, multicentre, single-blind, phase III clinical trial investigating the short-term treatment of HK, and the ongoing maintenance of normokalaemia. The study was carried out in two sequential parts over 12 weeks.
The treatment phase (Part A) was a single-blind single-arm trial of patiromer for 4 weeks. Patients were eligible for inclusion if they had stage 3 or 4 CKD, a serum potassium level of 5.1 to < 6.5mmol/L and were receiving a stable RAASi dose. At the time of screening, patients were assigned to receive a starting dose of 4.2 g twice daily or 8.4 g twice daily depending on the severity of HK. In this phase, RAASi doses were not adjusted; they were only discontinued if the potassium level was ≥ 6.5 mmol/L (≥ 5.1 mmol/L if taking the maximum permitted patiromer dose).
The withdrawal phase (Part B) was a placebo-controlled, single-blind, randomised withdrawal trial of patiromer for 8 weeks. The objective of the withdrawal phase was to evaluate the effect of withdrawing patiromer on serum potassium control and to assess whether long-term treatment with patiromer prevents the recurrence of HK.
2.2 Cost-Effectiveness Model
A Markov cohort model was developed to assess the health economic impact of patiromer therapy in comparison to standard of care (SoC) in controlling HK in advanced patients with CKD with and without HF. The model was designed to predict the natural history of CKD and HF and quantify the costs and benefits associated with the use of patiromer for serum potassium management from a payer perspective in Ireland. Chronic kidney disease and HF are chronic and progressive diseases associated with an increased risk of mortality. As such, a lifetime horizon was modelled in line with Health Information and Quality Authority technology assessment guidelines [43, 44]. A monthly cycle length was adopted and disease progression followed over a lifetime. The model was conducted and reported in line with recommendations from the Second Panel on Cost Effectiveness in Health and Medicine, including an inventory summarising the impact of patiromer on formal healthcare costs and patients’ health (Appendix A of the Electronic Supplementary Material [ESM]) [45].
2.3 Model Structure and Disease Progression
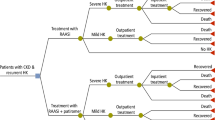
Patients enter the model (Fig. 1) with either CKD alone or CKD with HF. The progression of patients with CKD was modelled via transitions to more progressed CKD stages and eventually end-stage renal disease, comprising separate dialysis and transplant states. Similarly, the progression of HF in patients with CKD and HF was modelled via transitions between New York Heart Association (NYHA) classifications (I–IV) [46,47,48,49]. Both CKD and HF are modelled independently, with progression through health states in one not impacting progression through health states in the other, except for those exiting the model in the death health state. As a simplifying assumption, patients without HF at model initiation do not develop HF during the modelled time horizon. The starting distribution of patients is presented in Table 1, alongside baseline age and sex, whilst baseline rates of CKD and HF disease progression are described further in Appendix B of the ESM.
As the simulated cohort progresses through the model, the value of alternative treatments is captured through the occurrence of HK events, changes in RAASi use and treatment discontinuation. The likelihood of other events (MACE, hospitalisation and mortality) is also predicted and is impacted directly by a patient’s health state (i.e. CKD and HF) and by RAASi use and HK incidence (i.e. potassium level); baseline rates may be found in Appendix B of the ESM [50,51,52,53]. Major adverse cardiovascular events was defined as events of coronary heart disease, HF, ischemic stroke and peripheral arterial disease leading to hospitalisation. Hospitalisation was defined as any hospitalisation. The probabilities of MACE, hospitalisation and mortality, stratified by disease severity, are estimated for a patient with CKD only and a patient with HF only, and the higher of the two probabilities are then applied for the cohort with CKD and HF. In both cohorts, where all-cause mortality estimates from Irish-specific life tables exceeded mortality estimates based on comorbidities and RAASi use, the greater mortality rate was assumed.
2.4 Hyperkalaemia
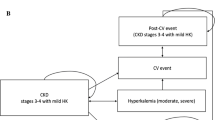
The occurrence of HK was categorised as a serum potassium level greater than 5 mmol/L, consistent with the definitions used in the OPAL-HK trial and widely accepted in the broader HK literature [34, 55]. Events were further stratified by severity (i.e. 5–5.5 mmol/L, 5.5–6 mmol/L and > 6 mmol/L). During the first 3 months of the modelled time horizon, incident HK events are predicted based on data from the OPAL-HK trial [34, 56]. For all subsequent months, annual rates of HK were obtained from Horne et al. and applied to the SoC arm. Hazard ratios relating to reduced (or increased) incidence in those receiving patiromer in subsequent years were obtained from the initial 3 months of data observed in the OPAL-HK trial and applied to the annual rates of HK obtained from Horne et al. [57]. Hyperkalaemia event rates are summarised in Table 2. Increased potassium levels negatively impact the incidence of MACE, hospitalisation and death (Fig. 2); the magnitude of these impacts is further described in Appendix B of the ESM.
Influence of renin-angiotensin-aldosterone system inhibitor (RAASi) use on disease progression and events. References below each box describe the baseline probabilities/rates; references alongside arrows describe the influence of one disease component on the other (e.g. in the form of hazard ratios, odds ratios or incidence rate ratios), with influences applied to the baseline probabilities rates. CKD chronic kidney disease, HF heart failure, HK hyperkalaemia, MACE major adverse cardiovascular event, SoC standard of care
2.5 RAASi Use
In both treatment arms, all patients are initiated in the model on RAASi and are assumed to be receiving a maximum dose. Down-titration to a sub-maximal dose, or discontinuation of RAASi treatment (from any dose) may occur. Renin-angiotensin-aldosterone system inhibitor use favourably impacts the progression of CKD and the incidence of MACE, hospitalisation and death (Fig. 2), with an unfavourable impact on the incidence of HK; the magnitude of these impacts is further described in Appendix B of the ESM [29, 46,47,48,49,50,51,52,53, 57,58,59,60].
The proportion of patients still receiving RAASi treatment at the end of the first month is specified for both arms and based on OPAL-HK trial data. For the patiromer arm, this proportion relates only to those that have achieved a response, with the remaining patients assumed to be receiving RAASi therapy in line with the SoC arm. Rates of RAASi discontinuation and down-titration are taken from the OPAL-HK trial for months 2 and 3 [54]. From month 4 onwards, potassium level-dependent RAASi discontinuation and down-titration rates were taken from Linde et al. and applied to the SoC arm [50]. Hazard ratios relating to reduced (or increased) rates of discontinuation/down-titration in those receiving patiromer in subsequent months were obtained from the initial 3 months of data observed in the OPAL-HK trial and applied to the rates from Linde et al. [22, 50]. To reflect the impermanent nature of RAASi treatment changes in clinical practice, patients could return to optimal RAASi use independent of their potassium level with a monthly probability of 3.51% [50]. Because of a lack of relevant data, patients who down-titrated RAASi use were assumed to not return to maximum use. Renin-angiotensin-aldosterone system inhibitor discontinuation and down-titration rates are summarised in Table 3.
2.6 Treatment
The model evaluates patiromer use against current SoC. It should be noted that modelling SoC is particularly challenging, owing to the considerable heterogeneity associated with HK pathogenesis, methods to correct and manage potassium levels (particularly non-pharmacological interventions, and variable levels of adherence to pharmacological methods) and patient responses to such interventions. As such, SoC has been defined consistently with the broad definitions used in the OPAL-HK study, where SoC can be considered short-term management for the correction of potassium and lifestyle interventions for the background maintenance of potassium (e.g. dietary intervention and modification of concomitant medications).
All patients initiated in the treatment arm were assumed to receive patiromer for at least 1 month. At the end of the first month, patients were stratified into those that do (60.93%) and do not (39.17%) respond to treatment. Within the patiromer arm, those that respond to treatment continue to receive patiromer with the associated event risks. Those that do not respond to patiromer cease treatment and incur the risk of events in line with SoC (i.e. assuming no legacy effect of patiromer treatment). For the SoC arm, treatment with SoC could not be discontinued. Beyond month 1, patients receiving patiromer could discontinue at a constant monthly rate of 10.33% based on the OPAL-HK trial, or if they reached end-stage renal disease; subsequently incurring an event risk in line with the SoC arm. Patients repeated treatment if their potassium levels were equal to or exceeded 5.5–6 mmol/L in subsequent months after discontinuation.
2.7 Costs and Utilities
Appendix C of the ESM summarises the direct medical costs (2019–20 Euros) applied to modelled health states and events. Irish-specific cost data were used where available and converted from GBP estimates where unavailable [61,62,63,64,65,66,67,68,69,70,71,72,73,74]. All costs were inflated to 2019/20 values. Appendix D of the ESM summarises the utilities (and disutilities) applied to modelled health states (and events). Utility estimates for each of the NYHA and CKD stages were measured with either the EQ-5D index [75, 76] or KDQOL [75] results and augmented by a recent National Institute for Health and Care Excellence technology appraisal [72]. To calculate the utility in a given state, the utilities of the respective NYHA classification and CKD stage were multiplied. Additionally, applicable transient events such as hospitalisation had their corresponding disutility added on when the event occurred, for instance if the previous example had a hospitalisation in a given period, their utility would be reduced to reflect this. Cost and utility values were discounted at a rate of 4% in line with Health Information and Quality Authority technology assessment guidelines [43].
2.8 Cost-Effectiveness Analysis
The model was used to evaluate the lifetime impact of patiromer use against SoC for the treatment of HK in patients with CKD with and without HF. Modelled outcomes focused on healthcare costs, life-years and quality-adjusted life-years (QALYs), with comparisons between treatments made using the incremental cost-effectiveness ratio.
A probabilistic sensitivity analysis, utilising 1000 simulations, was undertaken to evaluate uncertainty in clinical and economic outcomes. Patient characteristics and demographics were sampled using a normal distribution, probabilities and utility and disutility values were sampled using a beta distribution, and costs, hazard ratios and odds ratios were sampled using a gamma distribution. A deterministic sensitivity analysis, with parameters varied within sensible and parameter-appropriate bounds, was also undertaken to assess the impact of individual model parameters on model outcomes; the most influential and uncertain input parameters were incorporated in the analysis.
2.9 Model Validation
The quality of the model was assessed by means of face validity checks. Experienced staff, outside of the modelling team, tested the clinical appropriateness of the model by analysing differences between model output and relevant published figures. Digitised output from Kaplan–Meier curves [77] and life expectancy estimates [78], from relevant publications, were used to ensure survival trends by NYHA and CKD status aligned with expectations. Meanwhile, baseline population characteristics, from a real-world study by Linde et al. [50], were implemented to verify the clinical plausibility of MACE and mortality outcomes.
3 Results
3.1 Base-Case Analysis
Base-case cost-effectiveness results are presented in Table 4. Treatment with patiromer was associated with an increase in discounted life-years (8.62 vs 8.37) and an increase in discounted QALYs (6.15 vs 5.95). Incremental discounted costs were predicted at €4979 per patient, with an incremental cost-effectiveness ratio of €25,719 per QALY gained. Discounted incremental costs were predominantly driven by an initial increase in costs associated with patiromer treatment, increased costs of CKD and end-stage renal disease management because of an extension of life and reductions in RAASi titration costs over the patient’s lifetime, as a consequence of improved RAASi enablement.
Patients remained receiving patiromer treatment for an average of 7.7 months, with treatment associated with a reduction in the incidence of most clinical events and a delay in CKD disease progression. Per 1000 patients, patiromer was associated with 218 and 50 fewer HK events, when evaluating potassium levels at the 5.5–6 mmol/L and > 6 mmol/L levels, respectively. In comparison to SoC, patiromer was also associated with 165 fewer RAASi discontinuation episodes and 64 fewer RAASi down-titration episodes per 1000 patients, whilst being associated with an additional seven MACE and 204 hospitalisation events per 1000 patients. Subsequently, improvements in RAASi management enabled an overall increase in the time it took patients to reach RRT, with a similar number of incident dialysis and transplant episodes across arms (an additional six incident dialysis events and two incident transplantation events per 1000 patients in the patiromer arm).
Nevertheless, when comparing the annualised rate of adverse outcomes, patiromer was associated with lower overall rates of HK, hospitalisation, MACE, dialysis, RAASi discontinuation, RAASi down-titration and mortality (Fig. 3). Importantly, this demonstrates that the increased absolute adverse outcome incidence of MACE, hospitalisation, dialysis and transplantation in the patiromer arm comes as a consequence of an extension of life (i.e. as patients in the patiromer arm survive longer than those in the SoC arm, they are subject to a longer period of time over which adverse outcome incidence may be accrued). Such a relationship accounts for the greater RRT costs (Table 4) observed in the patiromer arm where, despite reductions in the rate of CKD progression and RRT incidence, overall event incidence was higher for patiromer because of an extension of life; a similar impact on hospitalisation costs is also observed.
3.2 Model Validation
Face validation of the model was carried out in two key stages; a comparison of NYHA-specific mortality from Briongos-Figuero et al. [77] and of CKD and HF (non-comorbid) mortality and MACE data using Linde et al. [50]. Patients with higher NYHA functional status had increasingly higher mortality rates, which is partially consistent with Briongos-Figuero et al. [77] Face validity was checked by ensuring that mortality in each NYHA status increased if the CKD status was higher, in line with clinical plausibility, although modelling both CKD and HF simultaneously may have reduced the proportional mortality difference between NYHA stages. Additionally, data in the academic literature were subject to inconsistency in baseline characteristics leading to potential limitations in comparability, for example, the study by Briongos-Figuero et al. did not age standardise NYHA subgroups.
Face validity of the estimated mortality and MACE output for both CKD with/without HF and HF with/without CKD was conducted using outcomes from Linde et al. [50]. As this publication was utilised in the internal calibration of the model, external validation of event output was not appropriate. The model predicted lower mortality and MACE outcomes than those observed in Linde et al., which is potentially owing to the model using a conservative clinical estimate of the baseline potassium level. In addition, the incidence rate ratio was not computed for validation purposes as the baseline model population characteristics in Linde et al. were not feasible to replicate.
3.3 Probabilistic Sensitivity Analysis
Probabilistic sensitivity analysis is presented in Figs. 4 and 5 and supports the conclusions of the base-case analysis. The average incremental discounted QALYs and costs were 0.192 and €5138 per patient, respectively, resulting in an incremental cost-effectiveness ratio of €26,752 (€14,561–€32,717)Footnote 1 [79] per QALY gained. At a willingness-to-pay threshold of €45,000 in Ireland, treatment with patiromer was estimated to have a 100% chance of cost effectiveness compared with SoC. One-way sensitivity analyses (Fig. 6) demonstrate that cost-effectiveness conclusions are relatively robust to changes in individual parameters, with results most sensitive to rates of discounting, the modelled time horizon, baseline patient age, the magnitude of the impact of RAASi use on CKD progression, and RAASi and treatment discontinuation.
4 Discussion
This is the first published study to evaluate the cost effectiveness of patiromer for the treatment of HK in patients with CKD with and without HF in Ireland. Prior to this study being undertaken, cost-effectiveness estimates for patiromer in Ireland have only been undertaken in the context of a National Centre for Pharmacoeconomics submission, completed in 2019, for which little methodological information is available. Cost-effectiveness estimates varied from €37,951 (company base case) to €117,396 (National Centre for Pharmacoeconomics preferred base case) per QALY gained. In an attempt to ensure any shortcomings of this previous analysis are addressed, we utilised a modelling approach previously adopted and accepted by the National Institute for Health and Care Excellence, and published in multiple journals, for analysing cost effectiveness in this indication, as a template to inform development of the de novo model described herein.
The results of this analysis demonstrate that the introduction of patiromer in the Irish healthcare setting may be associated with an improved quality of life and life expectancy, with only a marginal additional cost. Patiromer demonstrably offers particular benefit in terms of RAASi management, enabling the continuation of RAASi therapy. Such findings are particularly important given the lack of treatment options for the long-term management of HK in patients with CKD with and without HF, where the ability to maintain RAASi management is key for halting disease progression and the event incidence of these underlying chronic conditions.
Hyperkalaemia is common in Ireland and patients with elevated potassium serum levels often have an increased risk of recurrence [25, 26]. A retrospective observational study of 205,334 patients, who accessed the Irish healthcare system between 2006 and 2010, found the incidence of HK (>5 mmol/L) was 21% with a 30% risk of recurrent HK, whilst the incidence of moderate/severe HK (>5.5 mmol/L) was 5% with an 18% risk of recurrent HK [80]. Another retrospective study of 60,864 adult patients admitted to St James’ Hospital in Dublin between 2002 and 2012 assessed the relationship between HK incidence, hospital length of stay and in-hospital mortality [81]. Overall, HK (>5 mmol/L) was present in 4.9% of the patient population and elevated serum potassium levels were associated with an increased risk of death and hospital length of stay [81], highlighting the importance of maintaining normal serum potassium levels.
Patiromer offers a novel approach to HK management and has been shown to safely and effectively maintain normal serum potassium levels in patients with CKD and HK receiving RAASi treatment over a long duration [35, 82, 83], avoiding down-titration of RAASi therapy. It has also been shown to enable RAASi up-titration in patients with CKD with hypertension during the AMBER clinical trial [37, 38, 84]. In this 12-week trial, RAASi use induced a reduction from baseline in systolic blood pressure that was statistically significant in both patiromer and placebo arms. However, up-titration of RAASi use in patients receiving patiromer together with an increased rate of RAASi discontinuation in the placebo arm did not translate into differences in clinical outcomes, i.e. blood pressure, between the two groups. However, in 36% of patients who had discontinued RAASi treatment before the end of the trial, drug metabolites were detectable 3 weeks later. Hence, the long half-life of RAASi treatment, together with the short duration of the AMBER trial, most likely allowed patients who discontinued treatment to prolong the effects of RAASi therapy in the short term [37]. More recently, the long-term effects of patiromer on clinical outcomes in patients with HF, such as MACE and cardiovascular-related mortality, are being investigated in the phase IIIb DIAMOND clinical trial [85]. Initial results suggest that patients are able to maintain long-term potassium control, with a reduction in HK and prolonged optimised RAASi use [86].
Our results are in accordance with other European cost-effectiveness analyses, which indicate that patiromer offers value for money as a treatment option for HK in patients with CKD through RAASi enablement [87, 88]. A study from a Swedish perspective reported that patiromer had a 50% chance of being cost effective in patients with stage 3–4 CKD aged above 65 years, by enabling RAASi treatment, yielding an incremental cost-effectiveness ratio of €43,307 [88]. Similarly, another study evaluated patiromer use in patients with stages 3–4 CKD receiving RAASi treatment from an Austrian healthcare system perspective [87]. Authors reported a significant improvement in QALYs with 7 months in perfect health and an incremental cost-utility ratio of €18,979.23 in patients with CKD treated with patiromer versus no patiromer. Together with our data, these results suggest that patiromer, alongside RAASi therapy, should be considered for use in patients with HK with advanced CKD and warrants further cost-effectiveness analyses in other European countries to be undertaken.
Current guidelines for the management of persistent recurrence of HK are limited by the lack of safe and effective treatments. Calcium polystyrene sulfonate and sodium polystyrene sulfonate ion-exchange resins are commonly used to correct serum potassium levels; however, often patients develop serious gastrointestinal adverse events that limit their long-term use [89,90,91,92]. In addition, clinicians often advise patients with HK to limit their potassium intake; but this can often lead to poor adherence or adverse clinical outcomes resulting from the reduction in key vitamins, minerals and fibre required for a healthy diet [93, 94]. Loop diuretics lower potassium levels and are frequently used in patients with HF, however, their use in long-term HK management would require down-titration of treatment in order to avoid any adverse clinical outcomes associated with hypokalaemia; electrolyte imbalance, reduced renal function and symptomatic hypotension [95, 96]. Even though guidelines recommend a down-titration of loop diuretics, in reality, only a small percentage of patients receive this change in dose in the clinical setting [97].
Frequently, RAASi down-titration is implemented in the clinic as a treatment strategy for HK management in a patient with CKD with and without HF; however, this limits the clinical benefits of optimal RAASi therapy and is associated with worsening clinical outcomes [30,31,32,33] and increased healthcare costs [98]. As such, the availability of treatment options that are able to reduce potassium levels and enable the continuation of RAASi therapy is of great significance. However, given the uncertainty in the Irish hyperkalaemic management structure, there is potential scope for further optimisation of treatment strategies and guidelines. Such optimisation might be realised through consideration of short-term HK care, the targeting of particular subgroups with a high propensity for HK incidence or a closer examination of outcomes across differential potassium thresholds. Inherently, each of these aspects should be considered from both a clinical and economic perspective, with the research presented here providing an initial framework from which further research may be developed.
Despite demonstrating the robustness of cost-effectiveness conclusions through probabilistic and one-way sensitivity analyses, the analysis is subject to some limitations. First, we consider only the payer perspective in this analysis. Accurately reflecting the societal burden of patients with HK who have CKD with or without heart failure, given the lack of research on societal impacts in patients with such a complex mix of diseases, is inherently difficult. However, given the improvement in RAASi enablement associated with patiromer, and the associated reduction in the rate of hospitalisations, MACE and RRT incidence, it is anticipated that excluding the societal impact only serves to underestimate the value of patiromer and RAASi enablement, and thus is unlikely to bias conclusions. Next, it is acknowledged that the OPAL-HK trial design inherently introduces some uncertainty given that initially all patients receive patiromer for 4 weeks (part A) before introducing the placebo-controlled design for the subsequent 8 weeks (part B). As such, part B of the trial addressed stopping patiromer in people already receiving it whose HK had responded rather than starting patiromer in people who might benefit from it. Further, the short-term nature of the trial prohibited the observation of patients who were maintained on patiromer in the long term, necessitating the extrapolation of outcomes. Further, because of the lack of data available for the Irish population, some point estimates used were derived from other countries, such as the USA, which may not truly reflect the healthcare and ethnicity mix in Ireland. However, all due diligence was undertaken to ensure the best available data were sourced for optimal transferability of data.
A further key source of uncertainty is the background rate of incident HK. The OPAL-HK trial observed relatively high rates of HK in the second and third months of the trial, compared with published rates of HK recurrence [34]. Such an observation might suggest that a large proportion of HK events go undiagnosed in the real world, where patients are not monitored and followed up on a regular basis (unlike a trial setting). Under base-case model settings, an increased rate of HK recurrence would increase the re-initiation of patiromer, resulting in a greater reduction in adverse clinical outcomes. Finally, the core mechanism for estimating incremental costs and benefits in the model is reliant on patiromer influencing RAASi management, which in turn influences CKD disease progression and the incidence of other clinical events. Importantly, there exists a consensus in the published literature that RAASi management positively influences these outcomes; however, there is a degree of uncertainty as to the magnitude of such influence. With this in mind, and for the benefit of future studies of cost effectiveness in HK, it is important that further research be undertaken to garner consensus over the influence of RAASi management on such outcomes.
5 Conclusions
Through the development and implementation of a de novo disease progression and cost-effectiveness model, this study has demonstrated an economic case for the reimbursement of patiromer for the treatment of HK in patients with CKD with and without HF in Ireland. Patiromer was estimated to improve life expectancy and quality-adjusted life expectancy, whilst incurring marginal additional costs when compared with current SoC. Results are predominantly attributed to the ability of patiromer to enable the continuation of RAASi treatment whilst also reducing potassium levels. Economic conclusions were robust to sensitivity analyses.
Notes
The 95% confidence interval calculated using Fieller’s theorem.
References
Hougen I, Leon SJ, Whitlock R, Rigatto C, Komenda P, Bohm C, et al. Hyperkalemia and its association with mortality, cardiovascular events, hospitalizations, and intensive care unit admissions in a population-based retrospective cohort. Kidney Int Rep. 2021;6(5):1309–16.
Wiebe N, Klarenbach SW, Allan GM, Manns BJ, Pelletier R, James MT, et al. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64(2):230–8.
Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–21.
Nakhoul GN, Huang H, Arrigain S, Jolly SE, Schold JD, Nally JV Jr, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol. 2015;41(6):456–63.
Khanagavi J, Gupta T, Aronow WS, Shah T, Garg J, Ahn C, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251.
Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109(10):1510–3.
Hayes J, Kalantar-Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo-and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120(1):c8-16.
Korgaonkar S, Tilea A, Gillespie BW, Kiser M, Eisele G, Finkelstein F, et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study. Clin J Am Soc Nephrol. 2010;5(5):762–9.
Furuland H, McEwan P, Evans M, Linde C, Ayoubkhani D, Bakhai A, et al. Serum potassium as a predictor of adverse clinical outcomes in patients with chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC Nephrol. 2018;19(1):1–16.
Einhorn LM, Zhan M, Walker LD, Moen MF, Seliger SL, Weir MR, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–62.
Betts K, Woolley J, Xiang C, Tang W, Wu E. The cost of hyperkalemia in the United States. Kidney Int Rep. 2017;3(2):385–93.
Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis. 2017;70(1):21–9.
Chazard E, Dumesnil C, Beuscart R. How much does hyperkalemia lengthen inpatient stays? About methodological issues in analyzing time-dependant events. Stud Health Technol Inform. 2015;210:835–9.
Dunn JD, Benton WW, Orozco-Torrentera E, Adamson RT. The burden of hyperkalemia in patients with cardiovascular and renal disease. Am J Manag Care. 2015;21(15 Suppl.):s307–15.
Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–62.
Fitch K, Woolley JM, Engel T, Blumen H. The clinical and economic burden of hyperkalemia on Medicare and commercial payers. Am Health Drug Benefits. 2017;10(4):202–10.
Formiga F, Chivite D, Corbella X, Conde-Martel A, Arévalo-Lorido JC, Trullàs JC, et al. Influence of potassium levels on one-year outcomes in elderly patients with acute heart failure. Eur J Intern Med. 2019;60:24–30.
Fudim M, Grodin JL, Mentz RJ. Hyperkalemia in heart failure. J Am Heart Assoc. 2018;7(11): e009429.
Gasparini A, Evans M, Barany P, Xu H, Jernberg T, Ärnlöv J, et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant. 2019;34(9):1534–41.
Gilstrap LG, Fonarow GC, Desai AS, Liang L, Matsouaka R, DeVore AD, et al. Initiation, continuation, or withdrawal of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc. 2017;6(2): e004675.
Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307(2):157–64.
Linde C, Qin L, Bakhai A, Furuland H, Evans M, Ayoubkhani D, et al. Serum potassium and clinical outcomes in heart failure patients: results of risk calculations in 21 334 patients in the UK. ESC Heart Fail. 2019;6(2):280–90.
Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11(1):90–100.
Núñez J, Bayés-Genís A, Zannad F, Rossignol P, Núñez E, Bodí V, et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;137(13):1320–30.
Thomsen RW, Nicolaisen SK, Adelborg K, Svensson E, Hasvold P, Palaka E, et al. Hyperkalaemia in people with diabetes: occurrence, risk factors and outcomes in a Danish population-based cohort study. Diabet Med. 2018;35(8):1051–60.
Thomsen RW, Nicolaisen SK, Hasvold P, Garcia-Sanchez R, Pedersen L, Adelborg K, et al. Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish population-based cohort study. J Am Heart Assoc. 2018;7(11): e008912.
Yusuf AA, Hu Y, Singh B, Menoyo JA, Wetmore JB. Serum potassium levels and mortality in hemodialysis patients: a retrospective cohort study. Am J Nephrol. 2016;44(3):179–86.
Qin L, Horne L, Ashfaq A, MacLachlan S, Sinsakul M, LoCasale R. Health care resource utilization following hyperkalemia in adults with and without chronic kidney disease in the UK. In: ERA-EDTA Congress; 3–6 June 2017; Madrid.
Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–41.
Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl (2011). 2016;6(1):20–8.
Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl.):S212–20.
Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017;38(24):1883–90.
Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS. Physicians’ guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail. 2017;19(11):1414–23.
Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2014;372(3):211–21.
Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151–61.
Pitt B, Bakris GL, Bushinsky DA, Garza D, Mayo MR, Stasiv Y, et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17(10):1057–65.
Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394(10208):1540–50.
Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32(7):820–8.
National Institute for Health and Care Excellence. Patiromer for treating hyperkalaemia. NICE guidance 2020. https://www.nice.org.uk/guidance/ta623/resources/patiromer-for-treating-hyperkalaemia-pdf-82609015577029. Accessed 20 July 2022.
Center for Drug Evaluation and Research. Approval package for: Veltessa. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205739Orig1s000Approv.pdf. Accessed 20 July 2022.
European Medicines Agency. EPAR summary for the public: Veltessa. 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/veltassa. Accessed 20 July 2022.
ClinicalTrials.gov. A two-part, single-blind, phase 3 study evaluating the efficacy and safety of patiromer for the treatment of hyperkalemia (OPAL). NCT01810939. 2015. https://www.clinicaltrials.gov/ct2/show/NCT01810939. Accessed 20 July 2022.
Health Information and Quality Authority. Guidelines for the economic evaluation of health technologies in Ireland. 2019. https://www.hiqa.ie/sites/default/files/2019-07/HTA-Economic-Guidelines-2019.pdf. Accessed 20 July 2022.
National Institute for Health and Care Excellence. NICE DSU technical support documentation. 2020. 2021. http://nicedsu.org.uk/technical-support-documents/. Accessed 20 July 2022.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
Nuijten M, Andress DL, Marx SE, Curry AS, Sterz R. Cost effectiveness of paricalcitol versus a non-selective vitamin D receptor activator for secondary hyperparathyroidism in the UK: a chronic kidney disease Markov model. Clin Drug Investig. 2010;30(8):545–57.
Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–19.
NHS Blood and Transport. Annual report on kidney transplantation. 2019. https://www.odt.nhs.uk/statistics-and-reports/organ-specific-reports/. Accessed 20 July 2022.
Yao G, Freemantle N, Calvert MJ, Bryan S, Daubert J-C, Cleland JG. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J. 2007;28(1):42–51.
Linde C, Bakhai A, Furuland H, Evans M, McEwan P, Ayoubkhani D, et al. Real-world associations of renin-angiotensin-aldosterone system inhibitor dose, hyperkalemia, and adverse clinical outcomes in a cohort of patients with new-onset chronic kidney disease or heart failure in the United Kingdom. J Am Heart Assoc. 2019;8(22): e012655.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305.
Ford E, Adams J, Graves N. Development of an economic model to assess the cost-effectiveness of hawthorn extract as an adjunct treatment for heart failure in Australia. BMJ Open. 2012;2(5): e001094.
Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model prediction of survival in heart failure. Circulation. 2006;113(11):1424–33.
Vifor Pharma. OPAL-HK CSR. Data on file. 2014.
Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487–95.
Weir MR, Mayo MR, Garza D, Arthur SA, Berman L, Bushinsky D, et al. Effectiveness of patiromer in the treatment of hyperkalemia in chronic kidney disease patients with hypertension on diuretics. J Hypertens. 2017;35(Suppl. 1):S57-63.
Horne L, Ashfaq A, MacLachlan S, Sinsakul M, Qin L, LoCasale R, et al. Epidemiology and health outcomes associated with hyperkalemia in a primary care setting in England. BMC Nephrol. 2019;20(1):1–12.
Krogager ML, Eggers-Kaas L, Aasbjerg K, Mortensen RN, Køber L, Gislason G, et al. Short-term mortality risk of serum potassium levels in acute heart failure following myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2015;1(4):245–51.
Flather MD, Yusuf S, Køber L, Pfeffer M, Hall A, Murray G, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355(9215):1575–81.
UK Renal Association. UK Renal Registry 22nd annual report 2018. 2021. https://renal.org/about-us/who-we-are/uk-renal-registry. Accessed 20 July 2022.
National Institute for Health and Care Excellence. Clinical guideline [CG182]: chronic kidney disease in adults: assessment and management. 2016. https://www.nice.org.uk/guidance/cg182. Accessed 20 July 2022.
Healthcare Pricing Office. ABF 2020 admitted patient price list. 2020. https://www.hpo.ie/. Accessed 20 July 2022.
Kennedy C, Connaughton DM, Murray S, Ormond J, Butler A, Phelan E, et al. Home haemodialysis in Ireland. QJM. 2017;111(4):225–9.
Central Statistics Office. Irish life tables 2015-2017. 2020. https://www.cso.ie/en/releasesandpublications/er/ilt/irishlifetablesno172015-2017/. Accessed 20 July 2022.
Health Services Executive. Final National Renal Office End of Year Statistics, Dec 31 2020. https://www.hse.ie/eng/about/who/cspd/ncps/renal/resources/nro-end-of-year-statistics-2020.pdf. Accessed 20 July 2022.
Health Services Executive. System requirements for the development of organ donation and transplantation in the Republic of Ireland. 2013. https://www.hse.ie/eng/about/who/acute-hospitals-division/organ-donation-transplant-ireland/publications/. Accessed 20 July 2022.
Health Services Executive. Primary care reimbursement service statistical analysis of claims and payments 2019. 2019. https://www.sspcrs.ie/portal/annual-reporting/report/annual. Accessed 20 July 2022.
Health Services Executive. Primary Care Reimbursement service drug list, reimbursible items. 2019. Costs by the Health Service Executive (HSE) of Ireland. Individual drugs searched and median cost per mg calculated. Combination medications were excluded. https://www.sspcrs.ie/druglist/pub. Accessed 20 July 2022.
Curtis LA, Burns A. Unit costs of health and social care 2015. Personal Social Services Research Unit; 2015.
OECD. Purchasing power parity indices. 2021. https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm#indicator-chart. Accessed 20 July 2022.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
National Institute for Health and Care Excellence. Clinical guideline [TA599]: sodium zirconium cyclosilicate for treating hyperkalaemia. 2019. https://www.nice.org.uk/guidance/ta599/evidence. Accessed 20 July 2022.
Vifor Pharma. IQVIA (Uniphar wholesaler price). Data on file. 2020.
Vifor Pharma. IQVIA (United Drug wholesaler price). Data on file. 2020.
Lee AJ, Morgan CL, Conway P, Currie CJ. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin. 2005;21(11):1777–83.
Göhler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WS, et al. Utility estimates for decision-analytic modeling in chronic heart failure: health states based on New York Heart Association classes and number of rehospitalizations. Value Health. 2009;12(1):185–7.
Briongos-Figuero S, Estévez A, Pérez ML, Martínez-Ferrer JB, García E, Viñolas X, et al. Prognostic role of NYHA class in heart failure patients undergoing primary prevention ICD therapy. ESC Heart Fail. 2020;7(1):280–4.
Turin TC, Tonelli M, Manns BJ, Ravani P, Ahmed SB, Hemmelgarn BR. Chronic kidney disease and life expectancy. Nephrol Dial Transplant. 2012;27(8):3182–6.
Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Ann Rev Public Health. 2002;23(1):377–401.
Browne L, Austin S. Profiling hypokalaemic and hyperkalaemic states in the irish health system: testing rates, incidence and determinants. In: ERA-EDTA Congress; 2020; virtual.
Conway R, Creagh D, Byrne DG, O’Riordan D, Silke B. Serum potassium levels as an outcome determinant in acute medical admissions. Clin Med. 2015;15(3):239.
Rosano GMC, Seferovic P, Farmakis D, Filippatos G. Renin inhibition in heart failure and diabetes: the real story. Eu J Heart Fail. 2018;20(1):149–51.
Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–21.
Agarwal R, Rossignol P, Garza D, Mayo MR, Warren S, Arthur S, et al. Patiromer to enable spironolactone use in the treatment of patients with resistant hypertension and chronic kidney disease: rationale and design of the AMBER study. Am J Nephrol. 2018;48(3):172–80.
Bolaños JA, Seliger SL. Recurrent hyperkalemia in renin-angiotensin-aldosterone system inhibitor (RAASi) treatment: stuck between a rock and a hard place. Am Soc Nephrol. 2021;345–7.
Vifor Pharma. DIAMOND trial: Veltassa® enables patients to achieve long-term potassium control and optimized RAASi therapy. 2022. https://www.viforpharma.com/sites/vifor-corp/files/media/vifor-pharma-press-release-diamond-positive-topline-results-211221.pdf. Accessed 20 July 2022.
Walter E, Eichhober G, Voit M. PSY115: a cost-effectiveness analysis of RAASI-enabling patiromer for the treatment of hyperkalemia in Austria. Value Health. 2018;21:S456.
Widén J, Ivarsson M, Schalin L, Vrouchou P, Schwenkglenks M, Heimbürger O, et al. Cost-effectiveness analysis of patiromer in combination with renin-angiotensin-aldosterone system inhibitors for chronic kidney disease in Sweden. Pharmacoeconomics. 2020;38(7):747–64.
Cheng ES, Stringer KM, Pegg SP. Colonic necrosis and perforation following oral sodium polystyrene sulfonate (Resonium A/Kayexalate) in a burn patient. Burns. 2002;28(2):189–90.
Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159–61.
McGowan CE, Saha S, Chu G, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493–7.
Minford EJ, Hand T, Jones MC. Constipation and colonic perforation complicating calcium resonium therapy. Postgrad Med J. 1992;68(798):302.
Clegg DJ, Headley SA, Germain MJ. Impact of dietary potassium restrictions in CKD on clinical outcomes: benefits of a plant-based diet. Kidney Med. 2020;2(4):476–87.
Sussman EJ, Singh B, Clegg D, Palmer BF, Kalantar-Zadeh K. Let them eat healthy: can emerging potassium binders help overcome dietary potassium restrictions in chronic kidney disease? J Ren Nutr. 2020;30(6):475–83.
Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808–17.
Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Changes in loop diuretic dose and outcome after cardiac resynchronization therapy in patients with heart failure and reduced left ventricular ejection fractions. Am J Cardiol. 2017;120(2):267–73.
Kapelios CJ, Laroche C, Crespo-Leiro MG, Anker SD, Coats AJS, Díaz-Molina B, et al. Association between loop diuretic dose changes and outcomes in chronic heart failure: observations from the ESC-EORP Heart Failure Long-Term Registry. Eur J Heart Fail. 2020;22(8):1424–37.
Polson M, Lord TC, Kangethe A, Speicher L, Farnum C, Brenner M, et al. Clinical and economic impact of hyperkalemia in patients with chronic kidney disease and heart failure. J Manag Care Spec Pharm. 2017;23(4 Suppl.):S2-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Vifor Pharma Ltd who provided support for data analysis, model development and medical writing for this study, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Conflict of interest
Antonio Ramirez de Arellano and Carol M. Quinn are employees of Vifor Pharma Ltd. Thomas Ward, Tray Brown, Ruth D. Lewis and Melodi Kosaner Kliess are employees of HEOR Ltd. HEOR Ltd received fees from Vifor Pharma Ltd in relation to this study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data and material relevant to the analysis are presented in the outlined publication or supplementary material, with the exception of the model itself. The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript.
Code availability
The authors will respond to all enquiries regarding the details of the analysis should these not have been answered by the information provided in the methods section.
Author contributions
TW, ARdA, CMQ and TB conceptualised and designed the study. TW and MKK were responsible for the data analysis. All authors contributed to the interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ward, T., Brown, T., Lewis, R.D. et al. The Cost Effectiveness of Patiromer for the Treatment of Hyperkalaemia in Patients with Chronic Kidney Disease with and without Heart Failure in Ireland. PharmacoEconomics Open 6, 757–771 (2022). https://doi.org/10.1007/s41669-022-00357-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00357-z