Abstract
Objective
Our objective was to assess the cost effectiveness of apixaban versus edoxaban in the prevention of stroke and systemic embolism (SE) in patients with atrial fibrillation (AF) in Spain.
Methods
We customized a Markov model with ten health states to estimate the lifetime economic and clinical outcomes in 6-week cycles. The efficacy (clinical event rates per 100 patient-years) and safety data were derived from a pairwise indirect treatment comparison. The analysis was conducted from both the national health service (NHS) and societal perspectives, and included pharmaceutical costs (retail price plus value-added tax (VAT) and applicable national deductions) according to daily dosages (apixaban 10 mg (5 mg twice daily (bid)) and edoxaban 60 or 30 mg) and complications and disease-management costs, obtained from national databases. Utilities for quality-adjusted life-year (QALY) calculations reflected EuroQoL 5-Dimension scores in patients with AF. An annual discount rate of 3% was applied for costs (€, year 2019 values) and outcomes.
Results
In a 1000-patient cohort, apixaban 5 mg bid versus edoxaban 60 mg could avoid five strokes, six major bleedings and 29 clinically relevant non-major bleedings (CRNMBs). Compared with edoxaban 30 mg, apixaban could avoid 21 strokes and two SEs. An increase in bleedings was observed with apixaban (seven haemorrhagic strokes, 48 major bleedings and 17 CRNMBs). Apixaban yielded 0.04 additional QALYs compared with edoxaban 60 mg or 30 mg. Incremental costs/QALY were €9639.33 and €354.22 for apixaban versus edoxaban 60 mg and edoxaban 30 mg, respectively, from the NHS perspective and €7756.62 for apixaban versus edoxaban 60 mg from the societal perspective. Apixaban was dominant versus edoxaban 30 mg from the societal perspective. Sensitivity analyses confirmed the robustness of the model.
Conclusions
This study suggests that apixaban 5 mg bid is a cost-effective alternative to edoxaban for stroke prevention in the AF population in Spain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Apixaban (5 mg twice daily) is an effective therapy for the treatment of atrial fibrillation (AF), preventing events such as strokes and systemic embolism compared with edoxaban. |
Costs per patient treated with apixaban are similar to those in patients receiving edoxaban 60 mg or 30 mg daily. |
Apixaban could be considered a cost-effective option versus edoxaban for stroke prevention in patients with AF in Spain. |
1 Introduction
Atrial fibrillation (AF) is the most frequent cause of cardiac arrhythmia and is among the main causes of stroke and thromboembolic events [1]. It is associated with increased morbidity and mortality and high medical costs related to the substantial consumption of health resources for its management [2]. In Spain, the cost to the health system is up to €13,319 per patient with cardioembolic ischaemic stroke [3]. The total cost in patients admitted to stroke units equals €27,711 per patient, with up to €18,643 related to non-healthcare costs [4].
Although the current European clinical guidelines for patients with AF recommend anticoagulation therapy as a preventive measure for associated complications [5], more than 40% of affected Spanish patients are not treated according to these recommendations [6].
Traditionally, the elective treatment for stroke prevention in patients with AF has been based on the administration of a vitamin K antagonist (VKA), mainly acenocoumarol, with the consequent risk of developing treatment-related haemorrhage [7].
The discovery some years ago of direct-acting oral anticoagulants was a significant development. This drug class includes apixaban, dabigatran, rivaroxaban and, most recently, edoxaban. Their main advantage is that they do not require the continuous and permanent monitoring of international normalized ratio (INR) and dose adjustment to assure therapeutic drug concentrations that are required for VKAs.
The efficacy and safety of the direct-acting anticoagulant agents for stroke prevention in patients with AF have been demonstrated in many clinical trials: ARISTOTLE [8] (apixaban 5 mg twice daily (bid) vs. warfarin), AVERROES [apixaban vs. acetylsalicylic acid (ASA)] [9], RELY (dabigatran vs. warfarin) [10], ROCKET-AF (rivaroxaban vs. warfarin) [11] and ENGAGE-AF (edoxaban vs. warfarin) [12].
Several economic evaluations have provided additional information to the previous efficacy evidence for health decision makers. The efficiency of these drugs has been assessed in different settings and with different comparators. These studies include cost-effectiveness analyses of apixaban versus warfarin, acenocoumarol, ASA, dabigatran and rivaroxaban [13] and, most recently, a cost-utility analysis versus edoxaban in the UK [14]. Evaluations specifically for the Spanish setting include those for apixaban versus acenocoumarol [15], apixaban versus dabigatran [16] and apixaban versus rivaroxaban [17].
Since edoxaban, a new therapeutic option, is now available in Spain, this study aimed to assess the cost effectiveness of apixaban versus edoxaban for the prevention of stroke and systemic embolism (SE) in patients with AF in Spain to provide additional information for the medical decision-making process.
2 Material and Methods
A previously developed and validated Markov model [18, 19], simulating the evolution of AF, was customized to the Spanish setting. A panel of Spanish experts, comprising three clinicians (two cardiologists and one internist) and five health economists, who are among the authors of the present manuscript, validated the representativeness of the parameters used and provided information about the local management of patients. Clinicians completed online questionnaires, and a face-to-face meeting was held with all the experts and authors for discussion and to reach consensus related to the final input values.
A hypothetical cohort of 1000 patients eligible to be treated with apixaban or edoxaban was assessed to show potential events avoided in understandable figures. The clinical characteristics of this cohort, including average age (70 years), the proportion of females (35.3%) and CHADS2 average score (2.1), were defined based on the population included in the ARISTOTLE clinical trial [8], which assessed the efficacy and safety of apixaban and warfarin and were considered representative of Spanish patients with AF by the expert panel.
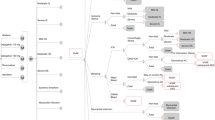
The model used for the present analysis represents the disease course, including ten main mutually exclusive health states: AF, ischaemic stroke, haemorrhagic stroke, other intracranial haemorrhages, other major bleedings, clinically relevant non-major bleedings, myocardial infarction (MI), SE, treatment discontinuation and death related to any cause different to the previously described clinical events. Based on the modified Rankin scale (mRS), the ischaemic or haemorrhagic strokes were categorized as mild (mRS 0–2), moderate (mRS 3–4) or severe (mRS 5).
The simulation was initiated with the whole cohort in the AF state. Throughout the simulation, to reflect a potential real-life patient evolution, the patients could remain in or transition through the previously described health states (Fig. 1). Patients remained in the AF state until stroke, bleeding, SE, MI, treatment discontinuation or death occurred.
Diagram for the Markov model. Red boxes represent health states. Triangles indicate which health state the patient enters after an event. Black lines represent the transitions allowed between the different health states. AC anticoagulation, CRNM clinically relevant non-major, HS haemorrhagic stroke, ICH intracranial haemorrhage, MI myocardial infarction, NVAF non-valvular atrial fibrillation, SE systemic embolism, w/o without
Transitions between the discrete health states were allowed in 6-week cycles (deliberately defined to capture the possibility of events related to AF occurring within such a short timeframe), with probabilities based on the likelihood of the different clinical events depending on the assigned treatment, reflecting efficacy and safety patterns for the assessed therapies.
Like the death state, the states of SE, ischaemic and haemorrhagic stroke (mild, moderate and severe) and MI were permanent states, with the rest being transient health states occurring for a maximum of 6 weeks before returning to the prior or moving to a subsequent health state.
A half-cycle correction approach was applied [20].
Hospitalizations for cardiovascular reasons other than those related to MIs or strokes were modelled in the background and not as health states.
2.1 Alternatives
This analysis is the last of a series of economic evaluations of apixaban compared with other anticoagulant treatments, such as acenocoumarol and other direct-acting oral anticoagulants (dabigatran and rivaroxaban), that were previously authorized and launched.
The present analysis focused on assessing apixaban compared with edoxaban, the latest direct-acting oral anticoagulant to become available on the Spanish market. Given the clinical data reported from the ENGAGE-AF clinical trial [12], high and low doses of edoxaban were assessed in the present model (60 or 30 mg daily, which is the recommended dose in patients with AF with moderate or severe renal impairment, low body weight ≤ 60 kg and/or concomitant use of cyclosporin, dronedarone, erythromycin or ketoconazole) [21].
The assessed dosage of apixaban was 5 mg bid [22], consistent with the dosage received by most of the patients in the ARISTOTLE trial [8] (< 4.7% of patients received the apixaban low dose, recommended for those aged ≥ 80 years or with body weight ≤ 60 kg or serum creatinine ≥ 1.5 mg/dL) and given that the published efficacy was reported for patients mainly treated with this dosage.
Aligned with the assumption used in previous customizations of the same model [14,15,16,17,18,19] and validated by the expert panel, the administration of ASA was considered a second-line treatment in patients who stopped or withdrew from the first-line therapy (any of the two drugs assessed).
2.2 Clinical Events
In the absence of head-to-head clinical trials, the clinical event rates per 100 patient-years were derived from a pairwise indirect treatment comparison analysis (detailed information can be found in a previous publication [14]), which was performed using the Bucher method [23]. In the indirect comparison, the relative hazard ratios (HRs) were estimated in relation to the existing common comparator, warfarin, from the apixaban and edoxaban clinical trials (ARISTOTLE [8] and ENGAGE-AF [12], respectively).
As in the previously published economic evaluation of apixaban versus edoxaban [14], the event rates and the HR (summarized in Table 1) were estimated with the rates observed per CHADS2 score level [CHADS2 0–1 (34.0%), CHADS2 2 (35.8%), CHADS2 ≥ 3 (30.2%)] and INR control based on the centre’s median time in therapeutic range.
For ASA, the event rates were derived from a subgroup of patients with prior VKA exposure from the AVERROES trial [9].
Case-fatality rates following the occurrence of each event derived from trials were applied. All-cause mortality excluding deaths attributable to stroke, bleeding, MI and SE were applied for patients in the AF health state to avoid double counting. Beyond 1.8 years (consistent with trial duration), mortality was modelled based on age- and sex-specific general mortality. The general mortality for males and females aged < and > 75 years were derived from published Spanish mortality data [24], which were modelled fitting a Gompertz survival function (details about parameters for the distribution function are available on request). The use of the Gompertz function instead of the raw data allowed a more refined estimation of the risk of mortality for every 6-week cycle (compared with yearly data on general life tables). In addition to background mortality, the model implemented adjustment factors, such as the HR, to reflect the potential increase in mortality rates associated with clinical events (stroke, MI, SE and bleedings). Since mortality due to strokes, MI, SE and bleedings was explicitly modelled at the occurrence of the event, increased mortality for patients with AF due to these causes was excluded from the calculation of the HR to avoid double counting.
Survival outcomes, estimated as life-years gained (LYG), were used to calculate quality-adjusted life-years (QALYs) by means of the utility values representing the patients’ preference for certain health states. In the present model, a basal utility associated with AF (0.727) and different utilities for the health states were used. Temporal decrements of utility in cases of complications were also applied (Table 2). All values for utilities and decrements were consistent with those previously used in international [14, 18, 19] and national [15,16,17, 25] publications. These data are referred to as the scores of the EuroQol 5-Dimensions (EQ-5D) questionnaire obtained in a sample of patients in the UK [26]. Further details about modelling can be found in previous publications [14, 18, 19], and additional information is also available on request.
2.3 Perspective, Discount Rate and Time Horizon
The analysis was carried out from two perspectives: that of the national health service (NHS) and the societal perspective.
The NHS perspective included direct medical costs related to drug acquisition, complication management, monitoring and AF patient follow-up. The societal perspective also included non-medical costs, but productivity costs were excluded due to lack of reliable data.
A 3% annual discount rate was applied to both costs and health outcomes, following the latest published recommendations for the development of economic evaluations in Spain [27].
A lifetime period was chosen for the time horizon. Coincident with previously published evaluations of apixaban compared with other alternatives performed in Spain [16, 17], this represents an average life expectancy of 80.4 years, which was considered by the expert panel to be applicable to patients with AF.
2.4 Resource Consumption and Costs
The estimation of total costs included the following direct medical costs: anticoagulant therapy acquisition, complication management in acute and maintenance phases, dyspepsia related to anticoagulant treatment, renal monitoring and clinical follow-up for AF. The acute phase, based on experts’ validation, was assumed to last 2 weeks, involving the time for hospital care and rehabilitation. Following this acute care period, maintenance phase costs were accumulated until the patient’s death.
The pharmaceutical costs for each of the two drugs assessed were calculated based on the daily dosages (apixaban 5 mg bid, edoxaban 30 mg and edoxaban 60 mg) stated on the summaries of product characteristics [21, 22] and the public retail prices [28] plus a VAT (4%), with the applicable deduction established by Royal Decree-Law 8/2010 [29].
The management costs of complications during the acute phase were derived from the average costs of diagnostic-related group official prices established by the autonomous regions [30].
The management costs of complications in maintenance, estimated as monthly costs, were obtained from several published Spanish sources [31,32,33,34,35].
The cost of dyspepsia related to anticoagulant treatment was included as a monthly clinical management cost obtained from the literature [36], considering the frequency (1.67%) observed in the ARISTOTLE trial [8] for apixaban and assuming an equivalent value for edoxaban. The renal monitoring cost was estimated as an annual cost, considering a yearly determination for each of the assessed therapies [37]. The cost of AF clinical follow-up was based on local clinical practice and validated by the expert panel, with a routine visit every 3 months assumed, regardless of the treatment chosen. Non-medical costs were obtained from the Spanish literature for both acute and maintenance costs [38], including societal costs related to the management of patient dependency, such as housing adaptation and informal care.
All costs are expressed in € (year 2019 values). When required, the consumer price index provided by the Statistic National Institute was applied. The unit costs used in the model are detailed in Table 3.
2.5 Cost-Effectiveness Analysis
The incremental cost-effectiveness ratio (ICER) in terms of cost per additional LYG and the incremental cost-utility ratio (ICUR) in terms of cost per additional QALY were estimated by means of differences in costs and health outcomes between the assessed therapies.
Although no official threshold is recognized by health authorities, the resulting ICUR was compared with the latest willingness-to-pay threshold estimated for the Spanish setting (€20,000/QALY) [39].
2.6 Sensitivity Analyses
Probabilistic sensitivity analysis (SA), to account for variability in outcomes due to statistical uncertainty in inputs, as well as one-way SA, to examine the effects of changes in key model parameters, was also performed.
A set of one-way SAs was performed by varying the base-case values of the parameters with uncertainty. The one-way SA comprised modifications of the discount rate (0%; 5%), mean patient age according to the interquartile range of patients in the ARISTOTLE trial [8] (63–77 years), economic inputs such as event management costs and resource costs (± 10% and ± 20%) and values concerning clinical inputs (event risks, HRs, death and case fatality rates and utilities) according to the 95% confidence intervals and standard deviations, if available, or assuming a standard error of 25% of the mean value.
The probabilistic SA consisted of simultaneous variation of all the potentially relevant parameters according to a distribution function previously assigned and adjusted to the data variability. The variations were conducted by running 2000 Monte Carlo iterations over the assessed 1000-patient cohort entering the model. The parametric functions applied were the beta distribution for utilities, the gamma distribution for costs and the HR of death and the log-normal distribution for the HR of clinical events.
3 Results
At the end of the simulation, for a cohort comprising 1000 patients with AF, apixaban 5 mg bid could additionally avoid five ischaemic strokes, one other intracranial haemorrhage, two MIs, six major bleedings and 29 clinically relevant non-major bleedings compared with those estimated for a 1000-patient cohort treated with edoxaban 60 mg daily. No differences were observed in the number of haemorrhagic strokes, recurrent haemorrhagic strokes or SEs. One additional recurrent ischaemic stroke in the 1000-patient cohort was reported for apixaban versus edoxaban 60 mg.
In comparison with 1000 patients treated with edoxaban 30 mg daily, the number of additional avoided events with apixaban 5 mg bid would be 21 ischaemic strokes, two SEs and eight MIs. There were no differences in terms of recurrent haemorrhagic strokes or other intracranial haemorrhages. In comparison with edoxaban 30 mg, apixaban was associated with more haemorrhagic strokes (seven additional cases) and other bleedings (48 additional major bleedings and 17 additional clinically relevant non-major bleedings) in the 1000-patient cohort. The complete detailed results are shown in Table 4.
In terms of quality-adjusted survival, apixaban would yield 6.924 QALYs per patient during the lifetime horizon compared with 6.882 QALYs yielded by edoxaban 60 mg and 6.844 QALYs by edoxaban 30 mg.
The estimated total cost per patient for each of the therapeutic alternatives from the NHS perspective would be €19,053 for apixaban, €18,651 for edoxaban 60 mg and €19,025 for edoxaban 30 mg.
From a societal perspective, the total cost per patient would be €32,620 for apixaban 5 mg bid compared with €32,297 and €34,034 for edoxaban 60 mg and 30 mg, respectively.
The ICUR from the NHS perspective was €9639 per QALY gained with apixaban versus edoxaban 60 mg and €354 per QALY gained with apixaban versus edoxaban 30 mg. From the societal perspective, the ICUR was €7757 per additional QALY with apixaban 5 mg bid versus edoxaban 60 mg daily, whereas apixaban 5 mg bid was a dominant alternative (more effective, less costly) versus edoxaban 30 mg daily.
Figure 2 is a graphical representation of the deterministic SA performed, including results for one-way SA over the discount rate, mean patient age, event and resource costs, and the ten clinical parameters that showed the greatest variations in ICUR versus the base-case results from the NHS perspective.
Results of the one-way SA for apixaban vs. a edoxaban 60 mg and b edoxaban 30 mg. The x-axis represents the resulting ICUR in terms of cost/QALY gained for apixaban vs. the comparator for the different one-way SAs. The y-axis lists the different SAs. A description of the SA mentioning the affected parameter is followed by the value used in the BC and values used in the SA. The vertical line between the dotted and grey areas of the bars indicates the ICUR resulting in the BC. The dotted area in the bars indicates that the ICUR of the SA with the first value specified in the parentheses following the SA description is below the ICUR estimated in the BC. The solid grey area in bars indicates that the ICUR of the SA with the second value specified in the parentheses following the SA description is above the ICUR estimated in the BC. ASA acetylsalicylic acid, BC base case, CRNMB clinically relevant non-major bleeding, CV cardiovascular, HR hazard ratio, ICH intracranial haemorrhage, ICUR incremental cost/utility ratio, QALY quality-adjusted life-year, SA sensitivity analysis. *Apixaban was a less effective option than edoxaban 60 mg
For apixaban compared with edoxaban 60 mg, the greatest variation was found for the stroke HR for edoxaban 60 mg, followed by the HR for the mortality trial period for edoxaban 60 mg. ICURs for the rest of the SAs were below €25,000/QALY. For apixaban compared with edoxaban 30 mg, stroke HR was also the parameter that had the widest variation, but the ICUR was lower than €15,000/QALY.
Probabilistic SA results are plotted in the cost-effectiveness planes in Fig. 3a for apixaban versus edoxaban 60 mg and Fig. 3b for apixaban compared with edoxaban 30 mg. In both comparisons, most of the iterations fall in the south-eastern quadrant, although a certain number of simulations appeared in the western quadrants, indicating cases when apixaban was a less effective option than edoxaban.
Cost-effectiveness planes for the probabilistic sensitivity analysis results from the national health service perspective: apixaban vs. a edoxaban 60 mg and b edoxaban 30 mg. The X-axis represents the incremental outcomes of apixaban vs. edoxaban in terms of additional QALYs, and the Y-axis represents the incremental costs. Each dot in the cost-effectiveness plane corresponds to the ICUR resulting in each of the 2000 Monte Carlo simulations performed in the probabilistic sensitivity analysis. The red line shows a willingness-to-pay threshold of €20,000 per QALY gained. ICUR incremental cost/utility ratio, QALY quality-adjusted life-year
Higher dispersion of the iterations was observed in the probabilistic SA for apixaban versus edoxaban 60 mg than for apixaban versus edoxaban 30 mg. The lower treatment discontinuation rates applied for edoxaban 30 mg (HR vs. apixaban 1.04; 95% confidence interval (CI) 0.96–1.13) than for edoxaban 60 mg (HR vs. apixaban 1.10; 95% CI 1.01–1.19) led to a decreased probability of apixaban being associated with negative incremental costs.
From the NHS perspective, 71% and 74% of the iterations of apixaban versus edoxaban 60 mg in the probabilistic SA yielded an ICUR below a willingness-to-pay threshold of €20,000/QALY and €30,000/QALY, respectively (Fig. 4a).
Cost-effectiveness acceptability curves with probabilistic sensitivity analysis results for apixaban vs. a edoxaban 60 mg, b edoxaban 30 mg. The X-axis reflects the willingness-to-pay threshold in terms of cost per additional QALY gained with apixaban vs. edoxaban. The Y-axis indicates the probability that a probabilistic sensitivity analysis iteration would fall below each threshold value and therefore reflects the proportion of cases when apixaban could be considered cost effective vs. edoxaban. ICUR incremental cost/utility ratio, QALY quality-adjusted life-year
Apixaban compared with edoxaban 30 mg was a cost-effective option in 86% and 88% of the iterations, considering willingness-to-pay thresholds of €20,000 and €30,000 per QALY gained, respectively (Fig. 4b).
4 Discussion
The present analysis complements a series of cost-effectiveness analyses performed for apixaban compared with other oral anticoagulant therapies for the prevention of stroke and SE in Spanish patients with AF [15,16,17, 25]. All of these economic evaluations were based on models with characteristics similar to those used in international evaluations [14, 18, 19].
In our analysis, apixaban was a cost-effective option in comparison with edoxaban 60 mg daily (€9639/QALY) and versus edoxaban 30 mg daily (€354/QALY) from the NHS perspective. An equivalent price was established for both dosages of edoxaban. Since edoxaban 30 mg was associated with a higher HR for stroke, MI and SE than edoxaban 60 mg, a lower ICUR was found for apixaban versus edoxaban 30 mg than for apixaban versus edoxaban 60 mg. Since this is the only economic evaluation assessing apixaban versus edoxaban in Spain, no comparison with other local results was possible. Our results are consistent with those obtained in previously published publications, which concluded that apixaban was cost effective versus dabigatran 110 mg [16], acenocoumarol [15], rivaroxaban [17] and ASA [25]. All ICURs estimated in these studies were also below the latest willingness-to-pay threshold estimated for the Spanish setting [39].
The economic evaluation of apixaban versus edoxaban in the UK [14] provided conclusions in the same kind of evaluation that was performed for the Spanish setting. Apixaban was a dominant option in comparison with low-dose edoxaban (30 mg) and a cost-effective alternative versus edoxaban 60 mg, with an ICUR of £6703/QALY gained (year 2012 values)
The present analysis is not exempt from limitations. The theoretical nature, inherent in every model, may not exactly represent usual clinical practice. The validity of analytic decision models depends on the quality of the data they are based on. In this case, the efficacy source included in the model was an indirect-comparison meta-analysis because of the lack of direct comparisons between the alternatives evaluated. This methodology, when developed with transparency and rigour, is widely recognized and accepted by health technology assessment agencies, such as the UK National Institute for Health and Care Excellence [40], and other scientific associations, such as the GENESIS group, belonging to the Spanish Society of Hospital Pharmacists [41]. The pivotal trials on which authorizations were based (ARISTOTLE [8] and ENGAGE-AF [12]) were considered the most robust studies to use as the source of individual efficacies. Additionally, the common comparator used in the trials (warfarin) allowed us to perform an indirect comparison by the Bucher method.
This indirect-comparison meta-analysis did not consider the potential influence of the patients’ baseline characteristics. Although patient age is a potential key driver, the results of the one-way SA indicated that age was not among the factors that had a major influence on the cost-effectiveness results.
The assumption of allowing one complication in each cycle is not representative of clinical practice, but it was necessary given the data availability. In the same sense, facing the absence of evidence for edoxaban, an equivalent frequency of dyspepsia was applied to both alternatives; again, the SA indicated that this assumption did not influence the results.
European Society of Cardiology guidelines [5] no longer recommend ASA monotherapy in patients with AF for stroke prevention, noting that it actually causes harm, incurs a higher bleeding risk and has very little effect on preventing thromboembolic events; however, its administration as a second-line treatment was implemented in the model for patients who stopped treatment. Given the lower cost of ASA, no significant influence on the results was anticipated. However, this assumption allowed us to model patients’ transitions, at least with the ASA efficacy reported in the AVERROES trial [9], faced with the lack of published evidence on the use of other direct-acting oral anticoagulants as second-line treatments.
Utility values were obtained from published literature given that the apixaban clinical studies for this indication did not include quality-of-life assessment questionnaires that allowed the collection of appropriate utility values for use in this economic evaluation. The values used in this analysis were obtained from patients in the UK because no specific data for the Spanish population were available. Utility values were endorsed by an expert panel that considered these data to be representative of Spanish patients. This supports the idea that utility values derived from health states (by using defined values from the EQ-5D questionnaire) do not differ between the general populations of different European countries [42].
The societal perspective included both medical and non-medical costs but not indirect costs because of the lack of reliable data about productivity loss in the Spanish AF population. This issue was not expected to have an impact on the results because of the advanced average age of the patients (70 years) in the simulated cohort.
Despite the limitations, both deterministic and probabilistic SAs confirmed the robustness of the model, as the vast majority of the estimated resulting ICURs did not show great variations with respect to the ICURs of the base cases.
5 Conclusion
This is the first economic evaluation to compare apixaban versus edoxaban in the Spanish setting. The methodology complied with international recommendations for the development of economic evaluations, and the results were concordant with previously published figures. Being the latest in a series of cost-effectiveness analyses for apixaban, it provides interesting information to be considered during the medical decision-making process. This study showed that apixaban 5 mg bid is a cost-effective option for the treatment of patients with AF in Spain compared with edoxaban 30 or 60 mg daily.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ferguson C, Inglis SC, Newton PJ, Middleton S, Macdonald PS, Davidson PM. Atrial fibrillation: stroke prevention in focus. Aust Crit Care. 2014;27(2):92–8.
Fitch K, Broulette J, Kwong WJ. The economic burden of ischemic stroke and major hemorrhage in medicare beneficiaries with nonvalvular atrial fibrillation: a retrospective claims analysis. Am Health Drug Benefits. 2014;7(4):200–9.
de Andrés-Nogales F, Vivancos Mora J, Barriga Hernández FJ, Díaz Otero F, Izquierdo Esteban L, Ortega-Casarrubios MÁ, Castillo Moreno L, Ximénez-Carrillo Rico Á, Martín Torres MP, Gómez-Escalonilla Escobar CI, Torres González C, de Salas-Cansado M, Casado Gómez MÁ, SotoÁlvarez J, Gil-Núñez A, Grupo de Investigación Estudio CODICE. Utilización de recursos sanitarios y costes asociados al manejo de los pacientes con infarto cerebral cardioembólico agudo en la Comunidad de Madrid: Estudio CODICE. Neurologia. 2015;30(9):536–44.
Alvarez-Sabín J, Quintana M, Masjuan J, Oliva-Moreno J, Mar J, Gonzalez-Rojas N, Becerra V, Torres C, Yebenes M, CONOCES Investigators Group. Economic impact of patients admitted to stroke units in Spain. Eur J Health Econ. 2017;18(4):449–58.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1–88.
de Andrés-Nogales F, Oyagüez I, Betegón-Nicolás L, Canal-Fontcuberta C, Soto-Álvarez J. Situación del tratamiento anticoagulante oral en pacientes con fibrilación auricular no valvular en España. Estudio REACT-AF Study. Rev Clin Esp. 2015;215(2):73–82.
Hussein A, Saliba W, Wazni OM. Evolving strategies to prevent stroke and thromboembolism in nonvalvular atrial fibrillation. Clevel Clin J Med. 2015;82(12 Suppl 2):S11–6.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S, AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
Pinyol C, Cepeda JM, Roldan I, Roldan V, Jimenez S, Gonzalez P, Soto J. A systematic literature review on the cost-effectiveness of apixaban for stroke prevention in non-valvular atrial fibrillation. Cardiol Ther. 2016;5(2):171–86.
Lip GY, Lanitis T, Kongnakorn T, Phatak H, Chalkiadaki C, Liu X, Kuznik A, Lawrence J, Dorian P. Cost-effectiveness of apixaban compared with edoxaban for stroke prevention in nonvalvular atrial fibrillation. Clin Ther. 2015;37(11):2476–2488.e27.
Barón Esquivias G, Escolar Albaladejo G, Zamorano JL, Betegón Nicolás L, Canal Fontcuberta C, De Salas-Cansado M, Rubio-Rodríguez D, Rubio-Terrés C. Análisis coste-efectividad de apixaban frente a acenocumarol en la prevención del ictus en pacientes con fibrilación auricular no valvular en España. Rev Esp Cardiol. 2015;68(8):680–90.
Betegón Nicolás L, Canal Fontcuberta C, Escolar Albaladejo G, De Salas Cansado M, Rubio-Rodríguez D, Rubio-Terrés C, Fernandez Gallego V. Cost-effectiveness analysis of apixaban versus dabigatran for prevention of stroke in patients with non-valvular atrial fibrillation in Spain. Eur J Clin Pharm. 2014;16(5):325–38.
Canal Fontcuberta C, Betegón Nicolás L, Escolar Albaladejo G, De Salas Cansado M, Rubio-Rodríguez D, Rubio-Terrés C. Análisis coste-efectividad de apixaban frente a rivaroxaban en la prevención del ictus en pacientes con fibrilación auricular no valvular en España. Pharmacoecon Span Res Artic. 2015;12:93–103.
Dorian P, Kongnakorn T, Phatak H, Rublee DA, Kuznik A, Lanitis T, Liu LZ, Iloeje U, Hernandez L, Lip GY. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35(28):1897–906.
Lip GY, Kongnakorn T, Phatak H, Kuznik A, Lanitis T, Liu LZ, Iloeje U, Hernandez L, Dorian P. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther. 2014;36(2):192–210.e20.
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–38.
Lixiana®. Summary of products characteristics. http://ec.europa.eu/health/documents/community-register/2018/20180726141621/anx_141621_en.pdf.
Eliquis®. Summary of Products Characteristics. https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_es.pdf.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91.
Instituto Nacional de Estadística. Tablas de mortalidad de la población de España. Serie 1975-2014. En: INEbase [Internet]. Madrid: Instituto Nacional de Estadística; 2014. https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176780&menu=ultiDatos&idp=1254735573175. Cited 4 Nov 2015.
Escolar Albadalejo G, Baron Esquivias G, Zamorano JL, Betegon Nicolas L, Canal Fontcuberta C, De Salas-Cansado M, Rubio-Rodríguez D, Rubio-Terrés C. Análisis coste-efectividad de apixaban frente al ácido acetilsalicílico en la prevención del ictus en pacientes con fibrilación auricular no valvular en España. Aten Primaria. 2016;48(6):394–405.
Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–4.
López-Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J. Spanish recommendations on economic evaluation of Health technologies. Eur J Health Econ. 2010;11(5):513–20. https://doi.org/10.1007/s10198-010-0244-4.
Consejo General de Colegios Oficiales de Farmacéuticos. Bot PLUS 2.0. Disponible en: https://botplusweb.portalfarma.com/. Accessed Feb 2019.
Real Decreto-Ley, de 20 de Mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. Disponible en: http://www.boe.es/boe/dias/2010/05/24/pdfs/BOE-A-2010-8228.pdf. Accessed Feb 2019.
Ministerio de Sanidad, Consumo y Bienestar Social, Instituto de información sanitaria. Registro de altas. CIE9 MC – CMBD 2016. En: Portal estadístico del Ministerio de Sanidad, Servicios Sociales e Igualdad [Internet]. Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad; 2013. https://www.mscbs.gob.es/estadEstudios/estadisticas/sisInfSanSNS/home.htm. Cited 9 Sep 2014.
Gómez-Outes A, Rocha E, Martínez-González J, Kakkar VV. Cost effectiveness of bemiparin sodium versus unfractionated heparin and oral anticoagulants in the acute and long-term treatment of deep vein thrombosis. Pharmacoeconomics. 2006;24:81–92.
Lamotte M, Piñol C, Brotons C, Annemans L, Guardiola E, Evers T, Kubin M. Evaluación económica del tratamiento con ácido acetilsalicílico en dosis bajas en la prevención primaria de enfermedades cardiovasculares. Rev Esp Cardiol. 2006;59(8):807–15.
Levy E, Gabriel S, Dinet J. The comparative medical costs of atherothrombotic disease in European countries. Pharmacoeconomics. 2003;21(9):651–9.
Mar J, Arrospide A, Begiristain JM, Larrañaga I, Elosegui E, Oliva J. The impact of acquired brain damage in terms of epidemiology, economics and loss in quality of life. BMC Neurol. 2011;11:46–56.
Monreal M, González-Rojas N, Vieta A, Wolowacz SE. Análisis económico de dabigatrán etexilato en prevención primaria del tromboembolismo venoso tras artroplastia total de cadera o rodilla. PharmacoEconomics Spa Res Art. 2009;6:126–45.
Ariza-Ariza R, Hernández-Cruz B, Navarro-Sarabia F. Rofecoxib frente a antiinflamatorios no esteroideos en el tratamiento de la artrosis: análisis coste-efectividad para España. Rev Clin Esp. 2004;204:457–65.
Oblikue Consulting. Base de datos de costes sanitarios. eSalud. Disponible en. http://www.oblikue.com. Accessed Feb 2019.
Beguiristain JM, Mar J, Arrazola A. Coste de la enfermedad cerebrovascular aguda. Rev Neurol. 2005;40:406–11.
Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–61.
NICE Decision Support Unit. Evidence Synthesis TSD Series. Disponible en. http://nicedsu.org.uk/technical-support-documents/evidence-synthesis-tsd-series/. Accessed Oct 2019.
Ortega Eslava A, Fraga Fuentes MD, Puigventós Latorre F, Santos-Ramos B, Clopés Estela A, Vilanova Boltó M. Comparaciones indirectas en los informes de evaluación de medicamentos en la web del grupo GENESIS de la SEFH. Farm Hosp. 2012;36:176–9.
Greiner W, Weijnen T, Nieuwenhuizen M, Oppe S, Badia X, Busschbach J, Buxton M, Dolan P, Kind P, Krabbe P, Ohinmaa A, Parkin D, Roset M, Sintonen H, Tsuchiya A, de Charro F. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ. 2003;4(3):222–31.
Author information
Authors and Affiliations
Contributions
CS, JLLS, JRGJ validated the parameters and provided data about local management of patients. IO and FAN performed the analysis. All the authors participated in the interpretation of the results. All authors had complete access to the data. IO and FAN drafted the manuscript. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This project received unrestricted financial support from Pfizer and Bristol-Myers Squibb S.A.U. Funding to pay the open access publication charges for this article was provided by Pfizer and Bristol-Myers Squibb S.A.U.
Conflict of interest
IO and FAN are employed at Pharmacoeconomics and Outcomes Research Iberia (PORIB), a consultant company that specializes in economic evaluation of health technologies and has received financial support from Bristol-Myers Squibb S.A.U. for the development of the present work. CS, JLLS and JRGJ have received an unrestricted grant, as members of an advisory board, to validate the model inputs. JSuarez and CP are full-time employees of Bristol-Myers Squibb S.A.U. JSoto is a full-time employee of Pfizer S.L.U.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Oyagüez, I., Suárez, C., López-Sendón, J.L. et al. Cost-Effectiveness Analysis of Apixaban Versus Edoxaban in Patients with Atrial Fibrillation for Stroke Prevention. PharmacoEconomics Open 4, 485–497 (2020). https://doi.org/10.1007/s41669-019-00186-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-019-00186-7