Abstract
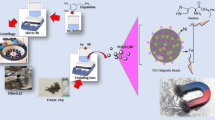
Protein A modified magnetic spheres (Fe3O4@SiO2-Protein A) with 22.7 emu/g saturation magnetization were prepared and characterized. These spheres had a diameter of 10 nm and was stable up to 229.2 °C, and can bond 19.694 mg/g of Protein A. The Fe3O4@SiO2-Protein A was firstly combined with oHSV-BJ-2-A antibody to capture oHSV from disease venom, and the amount of binding virus was 1.92 × 107 to 3.44 × 107 PFU/g. Fe3O4@SiO2-Protein A was modified with CD63 antibody, which was further fixed using dissuccinimide octylate (DSS) as a crosslinking agent. The prepared Fe3O4@SiO2-Protein A-CD63 Ab was useful in isolating and enriching exosomes from cell supernatant. Compared with commercial kits, Fe3O4@SiO2-Protein A-CD63 Ab demonstrated a better purification effect, which was successfully monitored using capillary electrophoresis. The total content of exosomes protein extracted using Fe3O4@SiO2-Protein A-CD63 was 3.4 mg/g, which was obviously higher than that reported in some studies. In addition, Fe3O4@SiO2-Protein A-CD63 Ab also showed its repeatability through bonding and elution of 5 cycles, which was effective in cost saving. These indicated Fe3O4@SiO2-Protein A-CD63 Ab had the potential for large-scale purification of exosomes in practical applications.







Similar content being viewed by others
Data availability
Data will be made available on request.
References
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–77.
Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai ZL, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–48.
Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79.
Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17.
Welch JL, Madison MN, Margolick JB, Galvin S, Gupta P, Martinez-Maza O, Dash C, Okeoma CM. Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci Rep. 2017;7:45034.
Kurywchak P, Tavormina J, Kalluri R. The emerging roles of exosomes in the modulation of immune responses in cancer. Genome Med. 2018;10(1):23–7.
Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab. 2017;19:137–46.
Zheng WQ, Li YX. Research progress of exosomes in the treatment of diabetes. Chin J Anal Lab. 2024;43(2):229–37.
Yuan L, Li J. Exosomes in Parkinson’s disease: current perspectives and future challenges. ACS Chem Neurosci. 2019;10:964–72.
Wang X, Xiang Z, Liu Y, Huang C, Pei Y, Wang X, Zhi H, Wong WH, Wei H, Ng IOL, Lee PPW, Chan WHS, Lau H, Tu W. Exosomes derived from Vδ2-T cells control Epstein-Barr virus-associated tumors and induce T cell antitumor immunity. Sci Transl Med. 2020;12:3426–41.
Gao L, Wang L, Wei Y, Wei Y, Krishnamurthy P, Walcott GP, Menasché P, Zhang J. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med. 2020;12:1318–28.
Kar R, Dhar R, Mukherjee S, Mukherjee S, Nag S, Gorai S, Mukerjee N, Mukherjee D, Vatsa R, Jadhav MC, Ghosh A, Devi A, Krishnan A, Thorat ND. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater Sci Eng. 2023;9:577–94.
Hu S, Li Z, Lutz H, Huang K, Su T, Cores J, Dinh PC, Cheng K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci Adv. 2020;6:1685–95.
Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044–61.
Shen M, Di K, He H, Xia Y, Xie H, Huang R, Liu C, Yang M, Zheng S, He N, Li Z. Progress in exosome associated tumor markers and their detection methods. Mol Biomed. 2020;1:3.
Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16:81–93.
Negahdaripour M, Vakili B, Nezafat N. Exosome-based vaccines and their position in next generation vaccines. Int Immunopharmacol. 2022;113: 109265.
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, Chen C, Veedu RN, Wang T. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–707.
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347.
Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine. 2017;13:2061–5.
Onodi Z, Pelyhe C, Terezia NC, Brenner GB, Almasi L, Kittel A, Mancek-Keber M, Ferdinandy P, Buzas EI, Giricz Z. Isolation of high-purity extracellular vesicles by the combination of iodixanol density gradient ultracentrifugation and bind-elute chromatography from blood plasma. Front Physiol. 2018;9:1479.
Chen BY, Sung CW, Chen C, Cheng CM, Lin DP, Huang CT, Hsu MY. Advances in exosomes technology. Clin Chim Acta. 2019;493:14–9.
Paolini L, Zendrini A, Di NG, Busatto S, Lottini E, Radeghieri A, Dossi A, Caneschi A, Ricotta D, Bergese P. Residual matrix from different separation techniques impacts exosome biological activity. Sci Rep. 2016;6:23550.
Gamez-Valero A, Monguio-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borras FE. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep. 2016;6:33641.
Cheruvanky A, Zhou H, Pisitkun T, Kopp JB, Knepper MA, Yuen PS, Star RA. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol-Renal. 2007;292:1657–61.
Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804.
Takov K, Teng IJ, Mayr M. Isolation of circulating extracellular vesicles by high-performance size-exclusion chromatography. Methods Mol Biol (Clifton, NJ). 2022;2504:31–40.
Iorember FM, Vehaskari VM. Uromodulin: old friend with new roles in health and disease. Pediatr Nephrol. 2014;29:1151–8.
Liu W, Ma Z, Kang X. Current status and outlook of advances in exosome isolation. Anal Bioanal Chem. 2022;414:7123–41.
Stranska R, Gysbrechts L, Wouters J, Vermeersch P, Bloch K, Dierickx D, Andrei G, Snoeck R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J Transl Med. 2018;16:1.
Park J, Park JS, Huang CH, Jo A, Cook K, Wang R, Lin HY, Van J, Li H, Min J, Wang L, Yoon G, Carter BS, Balaj L, Choi GS, Castro CM, Weissleder R, Lee H. An integrated magneto-electrochemical device for the rapid profiling of tumour extracellular vesicles from blood plasma. Nat Biomed Eng. 2021;5:678–89.
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24–46.
Mizutani K, Riyako T, Koji K, Taku K, Kengo H, Tomohiro T, Kensaku S, Hidetoshi E, Yasunori F, Kyojiro K, Masafumi I, Takashi D. Isolation of prostate cancer-related exosomes. Anticancer Res. 2014;34:3419–24.
Chinnappan R, Ramadan Q, Zourob M. An integrated lab-on-a-chip platform for pre-concentration and detection of colorectal cancer exosomes using anti-CD63 aptamer as a recognition element. Biosens Bioelectron. 2023;220: 114856.
Bautista MC, Bomati-Miguel O, Morales MD, Serna CJ, Veintemillas-Verdaguer S. Surface characterisation of dextran-coated iron oxide nanoparticles prepared by laser pyrolysis and coprecipitation. J Magn Magn Mater. 2005;293:20–7.
Sabani B, Brand M, Albert I, Inderbitzin J, Eichenseher F, Schmelcher M, Rohrer J, Riedl R, Lehmann S. A novel surface functionalization platform to prime extracellular vesicles for targeted therapy and diagnostic imaging. Nanomedicine. 2023;47: 102607.
Fang X, Chen C, Liu B, Ma Z, Hu Z, Li H, Gu H, Xu H. A magnetic bead-mediated selective adsorption strategy for extracellular vesicle separation and purification. Acta Biomater. 2021;124:336–47.
Kim H, Shin S. ExoCAS-2: rapid and pure isolation of exosomes by anionic exchange using magnetic beads. Biomedicines. 2021;9:28–39.
Nakai W, Yoshida T, Diez D, Diez D, Miyatake Y, Nishibu T, Imawaka N, Naruse K, Sadamura Y, Hanayama R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 2016;6:33935.
Tashiro M, Montelione GT. Structures of bacterial immunoglobulin-binding domains and their complexes with immunoglobulins. Curr Opin Struc Biol. 1995;5:471–81.
Wang L, Wan Y, Ma N, Zhou L, Zhao D, Yu J, Wang H, Lin Z, Qian W. Real-time kinetics and affinity analysis of the interaction between protein A and immunoglobulins G derived from different species on silica colloidal crystal films. Colloid Surf B Biointerfaces. 2022;219: 112839.
Nath N, Godat B, Benink H, Urh M. On-bead antibody-small molecule conjugation using high-capacity magnetic beads. J Immunol Methods. 2015;426:95–103.
Korodi M, Rakosi K, Baibarac M, Fejer SN. Reusable on-plate immunoprecipitation method with covalently immobilized antibodies on a protein G covered microtiter plate. J Immunol Methods. 2020;483: 112812.
Peng Z, Liu Z, Jiang Y, Dong Y, Shi L. In vivo interactions between Cyc2 and Rus as well as Rus and Cyc1 of Acidithiobacillus ferrooxidans during extracellular oxidization of ferrous iron. Int Biodeter Biodegr. 2022;173: 105453.
Fang G, Zhao W, Wang Y. Preparation and characterization of 3-glucidoxypropyltrim ethoxysilane films on core shell silica magnetic spheres. Appl Chem Ind. 2010;39:1190–3.
Mathieu M, Nevo N, Jouve M, Valenzuela JI, Maurin, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, Rubinstein E, Boncompain G, Perez F, Thery C. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12:4389–406.
Gong W, Gong R, Liu X, Liu X, Yao X, Liu H. Two methods to measure the epoxy value of five epoxy resins. Handong Chem Ind. 2021;23:92–4.
Yang C, Guan Y, Xing M, Jia G, Liu Z. Synthesis and protein immobilization of monodisperse magnetic spheres with multifunctional groups. React Funct Polym. 2006;66:267–73.
Redmile-Gordon MA, Armenise E, White RP, Hirsch PR, Goulding KW. A comparison of two colorimetric assays, based upon Lowry and Bradford techniques, to estimate total protein in soil extracts. Soil Biol Biochem. 2013;67:166–73.
Liu J, Song Q, Zheng W, Jia W, Jia H, Nan Y, Ren F, Bao J, Li Y. Preparation of boronic acid and carboxyl-modified molecularly imprinted polymer and application in a novel chromatography mediated hollow fiber membrane to selectively extract glucose from cellulose hydrolysis. J Sep Sci. 2022;45:2415–28.
Nath N, Godat B, Urh M. Antibody labeling with fluorescent dyes using magnetic protein A and protein G beads. Jove-J Vis Exp. 2016;115:54545.
Wu M, Jiang L, Zheng B. Application of magnetic immune microspheres in the purification of human serum albumin. Acta Pharmacol Sin. 2006;7:608–14.
Radi S, Ramdani A, Lekchiri Y, Morcellet M, Crini G, Janus L, Bacquet M. Immobilization of pyrazole compounds on silica gels and their preliminary use in metal ion extraction. New J Chem. 2003;27:1224–7.
Li G, Zhu N, Cheng J, Zhang Y, Yu Y, Zhang X, Yi Q, Wu Y. Dynamic biological interfaces functionalized fructose-responsive immunomagnetic beads for high-efficient and high-purity exosome enrichment. Mater Des. 2022;213: 110366.
Ma Z, Guan Y, Liu H. Superparamagnetic silica nanoparticles with immobilized metal affinity ligands for protein adsorption. J Magn Magn Mater. 2006;301:469–77.
Fan Z, Weng Q, Li Y, Zeng T, Wang J, Zhang H, Yu H, Dong Y, Zhao X, Li J. Accurate and rapid quantification of PD-L1 positive exosomes by a triple-helix molecular probe. Anal Chim Acta. 2023;1251: 340984.
Ouahabi OE, Salim H, Pero-Gascon R, Benavente F. A simple method for the analysis of extracellular vesicles enriched for exosomes from human serum by capillary electrophoresis with ultraviolet diode array detection. J Chromatogr A. 2021;1635: 461752.
Feng X, Iliuk A, Zhang X, Jia S, Shen A, Zhang W, Hu L, Tao W. Supramolecular exosome array for efficient capture and in situ detection of protein biomarkers. Anal Chem. 2023;95:2812–21.
Yang T, Wang X, Xie J. Effect of exosomes isolated by immunoprecipitation on α-syn content in the supernatant of PC12 cell. J Qingdao Univ. 2023;59:329–32.
Acknowledgements
Authors gratefully acknowledge the funding supports from National Natural Science Foundation of China (21605112).
Author information
Authors and Affiliations
Contributions
Wenqing Zheng and Pingyi Zheng conceived and designed the experiments; Wenqing Zheng, Pingyi Zheng, and Ran Zhao performed the experiments and analyzed the data; Xinyu Xu, Xiao Zhang, Xiaoqian Yuan helped to revise the paper; Ying Xu cultured cells; Youxin Li provided the concept of this research and managed all the experimental and writing process as the corresponding authors; Zichuan Liu guided the cell culture and revised the paper. All authors discussed the results, commented the paper and agreed to the published version of the paper.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zheng, W., Zheng, P., Zhao, R. et al. Fe3O4@SiO2-Protein A-oHSV/CD63 Ab for Capturing Virus and Exosomes. J. Anal. Test. (2024). https://doi.org/10.1007/s41664-024-00310-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41664-024-00310-5