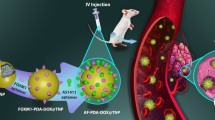
Abstract
Building self-assembly nanostructures is an important way to overcome the limitations of paclitaxel in tumor therapy. However, this strategy is also faced with challenges, such as difficulties in efficient release and the potential for drug resistance. Herein, we developed a near-infrared light-activatable melanized paclitaxel self-assembly nanoparticles for synergistic anti-tumor therapy. In this strategy, paclitaxel dimer prodrugs were synthesized and paclitaxel nanoparticles were obtained through self-assembly. Finally, the paclitaxel dimer nanoparticles were capped with polydopamine (PDA, melanoidin) and human serum albumin (HSA). The disulfide bonds in paclitaxel dimeric prodrug specifically respond to high concentrations of glutathione (GSH) and reactive oxygen species (ROS) in tumor cells. PDA enhances the biocompatibility of the drug molecules and imparts near-infrared photothermal conversion capability to the nano–self-assemblies. Both the in vitro and in vivo experiments demonstrated that this paclitaxel nanoprodrug exhibited enhanced tumor therapeutic efficacy under near-infrared light irradiation.







Similar content being viewed by others
Data Availability
The data and materials in current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Sun HT, Zhang Q, Li JC, Peng SJ, Wang XL, Cai R. Near-infrared photoactivated nanomedicines for photothermal synergistic cancer therapy. Nano Today. 2021;37: 101073.
Qiao J, Li XF, Qi L. Fluorescent polymer-modified gold nanobipyramids for temperature sensing during photothermal therapy in living cells. Chin Chem Lett. 2022;33(6):3193–6.
Huang QW, Zhu WS, Gao XY, Liu XP, Zhang ZJ, Xing BG. Nanoparticles-mediated ion channels manipulation: From their membrane interactions to bioapplications. Adv Drug Delivery Rev. 2023;195: 114763.
Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O, Kotowski K, Kulbacka J. Photodynamic therapy- mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–107.
Yang YJ, Zhang YF, Wang R, Rong X, Liu T, Xia X, Fan JL, Sun W, Peng XJ. A glutathione activatable pro-drug-photosensitizer for combined chemotherapy and photodynamic therapy. Chin Chem Lett. 2022;33(10):4583–6.
Zhang ZJ, Han QY, Lau JW, Xing BG. Lanthanide-doped upconversion nanoparticles meet the needs for cutting-edge bioapplications: recent progress and perspectives. ACS Mater Lett. 2020;2(11):1516–31.
Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, Weiss SA, Lo J, Fisher DE, Miao D, Van Allen E, Root DE, Sharpe AH, Doench JG, Haining WN. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547(7664):413–8.
Yang YH, Mao JW, Tan XL. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin J Nat Med. 2020;18(12):890–7.
Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665–7.
Govindan R, Szczesna A, Ahn MJ, Schneider CP, Mella PFG, Barlesi F, Han BH, Ganea DE, Von Pawel J, Vladimirov V, Fadeeva N, Lee KH, Kurata T, Zhang L, Tamura T, Postmus PE, Jassem J, O’Byrne K, Kopit J, Li MS, Tschaika MReck M. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–57.
Vokes EE, Haraf DJ, Stenson K, Stupp R, Malone D, Levin J, Weichselbaum RR. The role of paclitaxel in the treatment of head and neck cancer. Semin Oncol. 1995;22(5 Suppl 12):8–12.
Damen EWP, Wiegerinck PHG, Braamer L, Sperling D, de Vos D, Scheeren HW. Paclitaxel esters of malic acid as prodrugs with improved water solubility. Bioorg Med Chem. 2000;8(2):427–32.
Li C, Yu DF, Newman RA, Cabral F, Stephens LC, Hunter N, Milas L, Wallace S. Complete regression of well-established tumors using a novel water-soluble poly(l-Glutamic Acid)-paclitaxel conjugate1. Cancer Res. 1998;58(11):2404–9.
Mu L, Feng SS. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J Control Release. 2003;86(1):33–48.
Havel H, Finch G, Strode P, Wolfgang M, Zale S, Bobe I, Youssoufian H, Peterson M, Liu M. Nanomedicines: from bench to bedside and beyond. Aaps J. 2016;18(6):1373–8.
Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2018;14(1):93–126.
Wu X, Chen XM, Wang XY, He HS, Chen JW, Wu W. Paclitaxel-lipid prodrug liposomes for improved drug delivery and breast carcinoma therapy. Chin Chem Lett. 2023: 108756.
Tang L, Xie MY, Li J, Mei YJ, Cao YQ, Xiao QQ, Dong HJ, Zhang YH, Wang W. Leveraging nano-engineered mesenchymal stem cells for intramedullary spinal cord tumor treatment. Chin Chem Lett. 2023;34(5): 107801.
Sun XD, Wu QR, Li W, Gong XQ, Ge JY, Wu JB, Gao XH. Facile fabrication of drug-loaded PEGDA microcapsules for drug evaluation using droplet-based microchip. Chin Chem Lett. 2022;33(5):2697–700.
Fu SW, Li GT, Zang WL, Zhou XY, Shi KX, Zhai YL. Pure drug nano-assemblies: a facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharmacol Sin B. 2022;12(1):92–106.
Xu JW, Yan XG, Ge X, Zhang MZ, Dang XG, Yang Y, Xu F, Luo YL, Li GL. Novel multi-stimuli responsive functionalized PEG-based co-delivery nanovehicles toward sustainable treatments of multidrug resistant tumor. J Mater Chem B. 2021;9(5):1297–314.
Han QY, Lau JW, Do TC, Zhang ZJ, Xing BG. Near-infrared light brightens bacterial disinfection: recent progress and perspectives. ACS Appl Bio Mater. 2021;4(5):3937–61.
Jiao LZ, Li QS, Li CM, Gu JH, Liu XP, He SJ, Zhang ZJ. Orthogonal light-triggered multiple effects based on photochromic nanoparticles for DNA cleavage and beyond. J Mater Chem B. 2023;11(11):2367–76.
Fu QR, Zhang X, Song JB, Yang HH. Plasmonic gold nanoagents for cancer imaging and therapy. View. 2021;2(5):20200149.
Jing LH, Yang C, Zhang PS, Zeng JF, Li Z, Gao MY. Nanoparticles weaponized with built-in functions for imaging-guided cancer therapy. View. 2020;1(2): e19.
Pei Q, Hu XL, Zheng XH, Xia R, Liu S, Xie ZG, Jing XB. Albumin-bound paclitaxel dimeric prodrug nanoparticles with tumor redox heterogeneity-triggered drug release for synergistic photothermal/chemotherapy. Nano Res. 2019;12(4):877–87.
Yu SL, Zhang YJ, Wang X, Zhen X, Zhang ZH, Wu W, Jiang XQ. Synthesis of paclitaxel-conjugated β-cyclodextrin polyrotaxane and its antitumor activity. Angew Chem Int Ed. 2013;52(28):7272–7.
Sobczak M, Korzeniowska A, Goś P, Kolodziejski WL. Preparation and characterization of polyester- and poly(ester-carbonate)-paclitaxel conjugates. Eur J Med Chem. 2011;46(7):3047–51.
Wang YJ, Liu D, Zheng QC, Zhao Q, Zhang HJ, Ma Y, Fallon JK, Fu Q, Haynes MT, Lin G, Zhang R, Wang D, Yang X, Zhao L, He Z, Liu F. Disulfide bond bridge insertion turns hydrophobic anticancer prodrugs into self-assembled nanomedicines. Nano Lett. 2014;14(10):5577–83.
Sun BJ, Luo C, Zhang XB, Guo MR, Sun MC, Yu H, Chen Q, Yang WQ, Wang ML, Zuo SY, Chen PY, Kan QM, Zhang HT, Wang YJ, He ZG, Sun J. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat Commun. 2019;10(1):3211.
Kang WB, Ji YH, Cheng Y. Van der Waals force-driven indomethacin-ss-paclitaxel nanodrugs for reversing multidrug resistance and enhancing NSCLC therapy. Int J Pharm. 2021;603: 120691.
Zheng XY, Chen F, Zhang JX, Cai KY. Silica-assisted incorporation of polydopamine into the framework of porous nanocarriers by a facile one-pot synthesis. J Mater Chem B. 2016;4(14):2435–43.
Wang J, Pei Q, Xia R, Liu S, Hu XL, Xie ZG, Jing XB. Comparison of redox responsiveness and antitumor capability of paclitaxel dimeric nanoparticles with different linkers. Chem Mater. 2020;32(24):10719–27.
Rowinsky EK, Donehower RC. Paclitaxel (Taxol). N Engl J Med. 1995;332(15):1004–14.
Funding
This work was financially supported by National Natural Science Foundation of China (NSFC) (22007083); Zhejiang Provincial Innovation Center of Advanced Textile Technology and the Fundamental Research Funds of Shaoxing Keqiao Research Institute of Zhejiang Sci-Tech University (KYY2022004C); the Fundamental Research Funds of Shengzhou Innovation Research Institute of Zhejiang Sci-Tech University (SYY2023B000004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zhu, Q., Li, P., Huang, Q. et al. Near-Infrared Light-Activatable Melanized Paclitaxel Nano–Self-Assemblies for Synergistic Anti-tumor Therapy. J. Anal. Test. 7, 204–214 (2023). https://doi.org/10.1007/s41664-023-00262-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-023-00262-2