Abstract
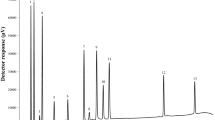
In this study, a specific and rapid high-performance liquid chromatography (HPLC) method has been developed and validated for the simultaneous determination of amoxicillin, lansoprazole, and levofloxacin in pharmaceuticals. Paracetamol was used as internal standard (IS) in the measurements. UV–Vis absorption spectra of the analytes and the IS were taken for the determination of suitable absorption wavelength of UV–Vis detector (diode array detector, DAD) in the HPLC instrument. A reverse-phase C18 column was used in the separation and determination of amoxicillin, lansoprazole, and levofloxacin together with the IS. The pharmaceutical analytes were quantified by the UV–Vis diode array detector in the HPLC using MeOH-0.01 M CH3COONH4 (70:30) as the mobile phase. The linear calibration curves of them were measured in the ranges of 15–40 mg/L, 2.5–15.0 mg/L, and 7.5–20.0 mg/L for amoxicillin, lansoprazole, and levofloxacin, respectively. Excellent calibration correlations (R2: 0.9942, 0.9997, and 0.9974) were obtained. The percentage recoveries of the amoxicillin, lansoprazole, and levofloxacin in commercial pharmaceuticals were obtained as 105.5%, 98.57%, and 102.5%, respectively. The results showed that amoxicillin, lansoprazole, and levofloxacin together with paracetamol IS could be separated and determined simultaneously with low LOD and LOQ values using the proposed HPLC method.





Similar content being viewed by others
References
Dousa M, Hosmanova R. Rapid determination of amoxicillin in premixes by HPLC. J Pharm Biomed Anal. 2005;7(2):373–7.
Vu DH, Do TG. Comparative study of RP-HPLC and UV spectrophotometric techniques for the simultaneous determination of amoxicillin and cloxacillin in capsules. J Young Pharmacist. 2010;2(2):190–5.
Nikam DS, Bonde CG, Surana SJ, Venkateshwarlu G, Dekate PG. Development and validation of RP-HPLC method for simultaneous estimation of amoxicillin trihydrate and flucloxacillin sodium in capsule dosage form. Int J Pharm Tech Res. 2009;1(3):935–9.
Shanmugasundaram P, Raj RK, Mohanrangan J, Devdass G, Arunadevi M, Maheswari R, Aanandhi MV. Simultaneous estimation of amoxicillin and flucloxacillin in its combined capsule dosage form by HPLC. Rasayan J Chem. 2009;2:57–60.
Borner K, Borner E, Lode H. Quantitative determination of lansoprazole in human serum by HPLC. Chromatographia. 1997;45(1):450–2.
Demir A, Aydın A. A new drug in the treatment of peptic ulcer disease: Lansoprazole. Turk J Gastroenterol. 1998;9(1):1–6.
Tadwee I, Ade P, Bhalerao P, Khan A, Shahi S. Development and validation of UV spectroscopy method for estimation of lansoprazole. World J Pharm Res. 2017;6(17):915–24.
Davis R, Bryson HM. Levofloxacin. Drugs. 1994;47:677–700.
Naveed S, Sultana N, Arayne MS, Dilshad H. A new HPLC method for the assay of levofloxacin and its application in drug–metal interaction studies. J Sci Innov Res. 2014;3(1):91–6.
Al-Momani IF. Flow injection spectrophotometric determination of the antibacterial levofloxacin in tablets and human urine. Anal Lett. 2006;39(4):741–50.
Nabi SA, Khan MA, Khowaja SN. Thin-layer chromatographic separation of penicillins on stannic arsenate-cellulose layers. Acta Chromatogr. 2006;16:164–72.
Storms ML, Stewart JT. Development of a reversed-phase liquid chromatographic method for the analysis of amoxicillin, metronidazole, and pantoprazole in human plasma using solid-phase extraction. J Liq Chromatogr RT. 2002;25:2433–43.
Luo WH, Ang CYW. Determination of amoxicillin residues in animal tissues by solid-phase extraction and liquid chromatography with fluorescence detection. J AOAC Int. 2000;83:20–5.
Dave JB, Banerjee SK. Spectrophotometric estimation of amoxycillin by reaction with diazotized primary aromatic amines. Indian J Pharm Sci. 1986;48:73–5.
Aktaş AH, Sarıdağ AM. Liquid Chromatographic–chemometric techniques for the simultaneous HPLC determination of lansoprazole, amoxicillin and clarithromycin in commercial preparation. J Chromatogr Sci. 2017;55(8):798–804.
Basavaiah K, Ramakrishna V, Somashekar BC. Spectrophotometric determination of lansoprazole in pharmaceuticals using bromate-bromide mixture based on redox and complexation reactions. Eclet Quim. 2007;32(1):57–64.
Özaltın N. Determination of lansoprazole in pharmaceutical dosage forms by two different spectroscopic methods. J Pharm Biomed Anal. 1999;20:599–606.
Krishna AM, Rajesh KS, Sudheer M, Kumar AK, Siva Kumar AVS, Sekhar GR, Nagarjuna S. New UV spectrophotometric method for the determination of lansoprazole in pharmaceutical dosage form and its application to protein binding study. J Pharm Res. 2011;4:1586–7.
Rahman N, Khan S. Experimental design approach in the optimization of potentiometric method for lansoprazole determination using lansoprazole-tungstate based ion-selective electrode. Ind Eng Chem Res. 2018;57(29):9351–61.
Wang H, Sun Y, Meng X, Yang B, Wang J, Yang Y, Gu J. Determination of lansoprazole enantiomers in dog plasma by column-switching liquid chromatography with tandem mass spectrometry and its application to a preclinical pharmacokinetic study. J Separ Sci. 2015;38(17):2960–7.
Cao S, Guo Y, Liao X, Guo Y, Ruan B, Zhao Y. Analysis of lansoprazole and its by products by liquid chromatography-electrospary ionization mass spectrometry. J. Inst. Anal. 2006;4:41–4.
Tivesten A, Folestad S, Schonbacher V, Svensson K. Nonaqueous capillary electrophoresis for the analysis of labile pharmaceutical compounds. Chromatographia. 1999;49:S7–11.
Oriquat G, Osman A, Abdul-Azim M, Abuhamdah S. Development and validation of a stability indicating spectrofluorometric method for the determination of lanzoprazole via its degradation product. J Appl Pharm Sci. 2014;4:57–61.
Yardımcı C, Özaltın C. Electrochemical studies and differential pulse polarographic analysis of lansoprazole in pharmaceuticals. Analyst. 2001;126:361–6.
Radi A. Anodic voltammetric assay of lansoprazole and omeprazole on a carbon paste electrode. J Pharm Biomed Anal. 2003;31:1007–12.
Basavaiah K, Ramakrishna V, Anilkumar UR, Udaya K. Spectrophotometric and high performance liquid–chromatographic determination of lansoprazole in pharmaceuticals. Indian J Chem Technol. 2006;13:549–54.
Reddy BP, Reddy GV. Validation and stability of RP-HPLC for the determination of lansoprazole in tablet dosage form and human plasma. Pharm Res. 2009;1:60–6.
Neckel U, Joukhoalor C, Frossard M, Jager W, Muller M, Mayer BX. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal Chim Acta. 2002;463:199–206.
Cheng FC, Tsai TR, Chen YF, Hung LC, Tsai TH. Pharmacokinetic study of levofloxacin in rat blood and bile by micro dialysis and high-performance liquid chromatography. J Chromatogr A. 2002;961:131–6.
Gonzalez JAO, Mochon MC, Rosq FTB. Spectrofluorimetric determination of levofloxacin in tablets, human urine and serum. Talanta. 2000;52:1149–56.
Du LM, Yang YQ, Wang QM. Spectrofluorometric determination of certain quinolone through charge transfer complex formation. Anal Chim Acta. 2004;516:237–43.
Huang JY, Bao T, Hu TX, Wen W, Zhang XH, Wang SF. Voltammetric determination of levofloxacin using a glassy carbon electrode modified with poly(o-aminophenol) and graphene quantum dots. Microchim Acta. 2017;184(1):127–35.
Altiokka G, Atkosar Z, Can NO. The determination of levofloxacin by flow injection analysis using UV detection, potentiometry, and conductometry in pharmaceutical preparations. J Pharm Biom Anal. 2002;30(3):881–5.
El-Brashy AM, Metwally ME, El-Scpai FA. Spectrophotometric determination of some fluoroquinolone antibacterials by binary complex formation with xanthene dyes. Farmaco. 2004;59(10):809–17.
Sastry CSP, Rao KR, Prasad DS. Extractive spectrophotometric determination of some fluoroquinolone derivatives in pure and dosage forms. Talanta. 1995;42(3):311–6.
Feng Z, Bian X, Zhi Z, Shen T. Study on the charge-transfer reaction between 7,7,8,8-tetracyanoquinodimethane and drugs. J Pharm Biom Anal. 1999;21(2):355–60.
Suliman FE, Sultan SM. Sequential injection technique employed for stoichiometric studies, optimization and quantitative determination of some fluoroquinolone antibiotics complexed with iron(III) in sulfuric acid media. Talanta. 1996;43(4):559–68.
Djabarouti S, Boselli E, Allauuchiche B, Ba B, Nguyen AT, Gordi JB, Bernadou JM, Saux MC, Breilh D. Determination of levofloxacin in plasma, bronchoalveolar lavage and bone tissues by high-performance liquid chromatography with ultraviolet detection using a fully automated extraction method. J Chromatogr B. 2004;799:165–72.
Czyrski A, Szałek E. An HPLC method for levofloxacin determination and its application in biomedical analysis. J Anal Chem. 2016;71(8):840–3.
Özdemir A, Korkmaz A. Comparative study of electrospray mass spectrometry and first derivative method and validation by HPLC method. J Food Drug Anal. 2007;15(2):118–25.
Joshi S. HPLC separation of antibiotics present in formulated and unformulated samples. J Pharm Biomed Anal. 2002;28(5):795–809.
Rele RV, Mali RN. Simultaneous determination of amoxicillin trihydrate and bromhexine hydrochloride in pharmaceutical dosage by reverse phase high performance liquid chromatography. Der Pharma Chem. 2013;5(1):273–8.
Chamseddin C, Jira TH. Comparison of the chromatographic behavior of levofloxacin, ciprofloxacin and moxifloxacin on various HPLC phases. Die Pharmazie-Int J Pharm Sci. 2011;66(4):244–8.
Miura M, Tada H, Suzuki T. Simultaneous determination of lansoprazole enantiomers and their metabolites in plasma by liquid chromatography with solid-phase extraction. J Chromatogr B. 2004;804(2):389–95.
Uddin MN, Das S, Khan SH, Shill SK, Bhuiyan HR, Karim R. Simultaneous determination of amoxicillin and chloramphenicol and their drug interaction study by the validated UPLC method. J Taibah Univ Sci. 2016;10(5):755–65.
Mohammadi A, Rezanour N, Dogaheh MA, Bidkorbeh FG, Hashem M, Walker RB. A stability-indicating high performance liquid chromatographic (HPLC) assay for the simultaneous determination of atorvastatin and amlodipine in commercial tablets. J Chromatogr B. 2007;846(1–2):215–21.
Sabry SM, Abdel-Hay MH, Belal TS, Mahgoub AA. Development and validation of HPLC-DAD method for the simultaneous determination of amoxicillin, metronidazole and rabeprazole sodium. Application to spiked simulated intestinal fluid samples. Ann Pharm Fr 2015;73(5):351–60.
González JO, Mochón MC, de La Rosa FB. Simultaneous determination of cefepime and the quinolones garenoxacin, moxifloxacin and levofloxacin in human urine by HPLC–UV. Microchim Acta. 2005;151(1–2):39–45.
Smerikarova M, Bozhanov S, Maslarska V. Development and validation of a RP-HPLC method to quantify amoxicillin, tinidazole, esomeprazole and lansoprazole in a mixture. Ind J Pharm Sci. 2020;81(6):1122–7.
Noubarani M, Keyhanfar F, Motevalian M, Mahmoudian M. Improved HPLC method for determination of four PPIs, omeprazole, pantoprazole, lansoprazole and rabeprazole in human plasma. J Pharm Pharm Sci. 2010;13(1):1–10.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gülfen, M., Canbaz, Y. & Özdemir, A. Simultaneous Determination of Amoxicillin, Lansoprazole, and Levofloxacin in Pharmaceuticals by HPLC with UV–Vis Detector. J. Anal. Test. 4, 45–53 (2020). https://doi.org/10.1007/s41664-020-00121-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-020-00121-4