Abstract
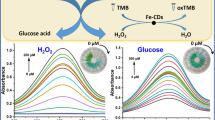
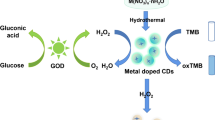
Nanozymes have become attractive in analytical and biomedical fields, mainly because of their low cost, long shelf life, and less environmental sensitivity. Particularly, nanozymes formed from nanomaterials having high surface area and rich active sites are interesting since their activities can be tuned through carefully controlling their size, morphology, and surface properties. This review article focuses on preparation of carbon dots (C dots) possessing peroxidase-like activity and their analytical applications. We highlight the important roles of the oxidation states and surface residues of C dots and their nanocomposites with metal, metal oxides, or metal sulfides playing on determining their specificity and sensitivity toward H2O2. Examples of C dot nanozymes (CDzymes) for developing sensitive and selective absorption, fluorescence, and electrochemical sensing systems in the presence of substrates are presented to show their potential in analytical applications. For example, CDzymes couple with glucose oxidase and cholesterol oxidase are specific and sensitive for quantitation of glucose and cholesterol, separately, when using 3,3′,5,5′-tetramethylbenzidine (TMB) as the signal probe. This review article concludes with possible strategies for enhancing and tuning the catalytic activity of CDzymes.
Article Highlights
-
Carbon dot nanozymes (CDzymes) possess peroxidase-like activity.
-
Low-cost and stable CDzymes based sensing systems are sensitive for quantitation of hydrogen peroxide.
-
CDzymes in conjunction with various enzymes have been used for quantitation of important analytes.








Similar content being viewed by others
Change history
23 March 2020
The publisher has retracted this article [1] due to an operational error during the publication process. The authors agree with this retraction.
References
Rocchitta G, Spanu A, Babudieri S, Latte G, Madeddu G, Galleri G, et al. Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sens Basel. 2016;16:780.
Privman M, Guz N, Katz E. Enzyme-logic digital biosensors for biomedical applications. Int J Unconv Comput. 2018;13:435–76.
Azevedo AM, Martins VC, Prazeres DMF, Vojinović V, Cabral JMS, Fonseca LP. Horseradish peroxidase: a valuable tool in biotechnology. Biotechnol Annu Rev. Elsevier. 2003;9:199–247.
Lin YH, Ren JS, Qu XG. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res. 2014;47:1097–105.
Zhou WH, Ding JS, Liu JW. Theranostic DNAzymes. Theranostics. 2017;7:1010–25.
Wang XY, Hu YH, Wei H. Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg Chem Front. 2016;3:41–60.
Zhou YB, Liu BW, Yang RH, Liu JW. Filling in the gaps between nanozymes and enzymes: challenges and opportunities. Bioconjugate Chem. 2017;28:2903–9.
Liu BW, Liu JW. Surface modification of nanozymes. Nano Res. 2017;10:1125–48.
Golchin J, Golchin K, Alidadian N, Ghaderi S, Eslamkhah S, Eslamkhah M, et al. Nanozyme applications in biology and medicine: an overview. Artif Cell Nanomed B. 2017;45:1069–76.
Yan XY. Nanozyme: a new type of artificial enzyme. Prog Biochem Biophys. 2018;45:101–4.
Li SR, Huang YC, Liu JR, Wang EK, Wei H. Nanozymes in analytical chemistry: from in vitro detection to live bioassays. Prog Biochem Biophys. 2018;45:129–47.
Huang YY, Lin YH, Pu F, Ren JS, Qu XG. The current progress of nanozymes in disease treatments. Prog Biochem Biophys. 2018;45:256–67.
Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060–93.
Peng YH, Wang ZY, Liu WS, Zhang HL, Zuo W, Tang HA, et al. Size- and shape-dependent peroxidase-like catalytic activity of mnfe2o4 nanoparticles and their applications in highly efficient colorimetric detection of target cancer cells. Dalton Trans. 2015;44:12871–7.
Lien CW, Huang CC, Chang HT. Peroxidase-mimic bismuth-gold nanoparticles for determining the activity of thrombin and drug screening. Chem Commun. 2012;48:7952–4.
Li CL, Huang CC, Chen WH, Chiang CK, Chang HT. Peroxidase mimicking DNA-gold nanoparticles for fluorescence detection of the lead ions in blood. Analyst. 2012;137:5222–8.
Lien CW, Chen YC, Chang HT, Huang CC. Logical regulation of the enzyme-like activity of gold nanoparticles by using heavy metal ions. Nanoscale. 2013;5:8227–34.
Hsu CL, Lien CW, Wang CW, Harroun SG, Huang CC, Chang HT. Immobilization of aptamer-modified gold nanoparticles on biocl nanosheets: tunable peroxidase-like activity by protein recognition. Biosens Bioelectron. 2016;75:181–7.
Ju Y, Kim J. Dendrimer-encapsulated Pt nanoparticles with peroxidase-mimetic activity as biocatalytic labels for sensitive colorimetric analyses. Chem Commun. 2015;51:13752–5.
Zhou NA, Zou SY, Zou L, Shen RD, Zhou YM, Ling LS. Peroxidase-like activity of palladium nanoparticles on hydrogen-bond supramolecular structures over a broader pH range and their application in glucose sensing. Can J Chem. 2019;97:317–23.
Jiang H, Chen ZH, Cao HY, Huang YM. Peroxidase-like activity of chitosan stabilized silver nanoparticles for visual and colorimetric detection of glucose. Analyst. 2012;137:5560–4.
Wei H, Wang E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem. 2008;80:2250–4.
Wang WJ, Wu YH, Lin XL, Chen W, Liu AL, Peng HP. Synthesis of Au-WS2 nanocomposites and study on its peroxidase mimic activity. Chin J Anal Chem. 2018;46:1545–51.
Hsu C-L, Lien C-W, Harroun SG, Ravindranath R, Chang H-T, Mao J-Y, et al. Metal-deposited bismuth oxyiodide nanonetworks with tunable enzyme-like activity: sensing of mercury and lead ions. Mater Chem Front. 2017;1:893–9.
Asati A, Santra S, Kaittanis C, Nath S, Perez JM. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Edit. 2009;48:2308–12.
Wan Y, Qi P, Zhang D, Wu JJ, Wang Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens Bioelectron. 2012;33:69–74.
Sun JH, Li CY, Qi YF, Guo SL, Liang X. Optimizing colorimetric assay based on V2O5 nanozymes for sensitive detection of H2O2 and glucose. Sens Basel. 2016;16:584.
Hsu KI, Lien CW, Lin CH, Chang HT, Huang CC. Immobilization of iron hydroxide/oxide on reduced graphene oxide: peroxidase-like activity and selective detection of sulfide ions. RSC Adv. 2014;4:37705–13.
Wu CW, Harroun SG, Lien CW, Chang HT, Unnikrishnan B, Lai IPJ, et al. Self-templated formation of aptamer-functionalized copper oxide nanorods with intrinsic peroxidase catalytic activity for protein and tumor cell detection. Sensor Actuat B-Chem. 2016;227:100–7.
Song W, Zhao B, Wang C, Ozaki Y, Lu XF. Functional nanomaterials with unique enzyme-like characteristics for sensing applications. J Mater Chem B. 2019;7:850–75.
Shin HY, Park TJ, Kim MI. Recent research trends and future prospects in nanozymes. J Nanomater. 2015. https://doi.org/10.1155/2015/756278.
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–83.
Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan X, Cai W. Nanozyme: new horizons for responsive biomedical applications. Chem Soc Rev. 2019. https://doi.org/10.1039/c8cs00718g.
Cardenas-Benitez B, Djordjevic I, Hosseini S, Madou MJ, Martinez-Chapa SO. Review-covalent functionalization of carbon nanomaterials for biosensor applications: an update. J Electrochem Soc. 2018;165:B103–17.
Yang ZB, Ren J, Zhang ZT, Chen XL, Guan GZ, Qin LB, et al. Recent advancement of nanostructured carbon for energy applications. Chem Rev. 2015;115:5159–223.
Jariwala D, Sangwan VK, Lauhon LJ, Marks TJ, Hersam MC. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem Soc Rev. 2013;42:2824–60.
Notarianni M, Liu JZ, Vernon K, Motta N. Synthesis and applications of carbon nanomaterials for energy generation and storage. Beilstein J Nanotech. 2016;7:149–96.
Chen TH, Chang HT. Stable and photoswitchable carbon-dot liposome. ACS Appl Mater Inter. 2017;9:44259–63.
Gopi CVVM, Ravi S, Rao SS, Reddy AE, Kim HJ. Carbon nanotube/metal-sulfide composite flexible electrodes for high-performance quantum dot-sensitized solar cells and supercapacitors. Sci Rep UK. 2017;7:46519.
Yang ZS, Chen CY, Liu CW, Li CL, Chang HT. Quantum dot-sensitized solar cells featuring CuS/CoS electrodes provide 4.1% efficiency. Adv Energy Mater. 2011;1:259–64.
Periasamy AP, Ravindranath R, Roy P, Wu WP, Chang HT, Veerakumar P, et al. Carbon-boron core-shell microspheres for the oxygen reduction reaction. J Mater Chem A. 2016;4:12987–94.
Wang ZH, Shen DK, Wu CF, Gu S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018;20:5031–57.
Wang QQ, Wei H, Zhang ZQ, Wang EK, Dong SJ. Nanozyme: an emerging alternative to natural enzyme for biosensing and immunoassay. Trend Anal Chem. 2018;105:218–24.
Wu JJX, Wang XY, Wang Q, Lou ZP, Li SR, Zhu YY, et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev. 2019;48:1004–76.
Huang YY, Ren JS, Qu XG. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119:4357–412.
Roy P, Chen PC, Periasamy AP, Chen YN, Chang HT. Photoluminescent carbon nanodots: synthesis, physicochemical properties and analytical applications. Mater Today. 2015;18:447–58.
Atabaev TS. Doped Carbon dots for sensing and bioimaging applications: a minireview. Nanomater Basel. 2018;8:342.
Zuo PL, Lu XH, Sun ZG, Guo YH, He H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta. 2016;183:519–42.
Zhang J, Yu SH. Carbon Dots: large-scale synthesis, sensing and bioimaging. Mater Today. 2016;19:382–93.
Zhan J, Geng BJ, Wu K, Xu G, Wang L, Guo RY, et al. A solvent-engineered molecule fusion strategy for rational synthesis of carbon quantum dots with multicolor bandgap fluorescence. Carbon. 2018;130:153–63.
Zhang WF, Xu T, Liu ZW, Wu NL, Wei MD. Hierarchical TiO2-X imbedded with graphene quantum dots for high-performance lithium storage. Chem Commun. 2018;54:1413–6.
Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Georgakilas V, Giannelis EP. Photoluminescent carbogenic dots. Chem Mater. 2008;20:4539–41.
Zheng LY, Chi YW, Dong YQ, Lin JP, Wang BB. Electrochemiluminescence of water-soluble carbon nanocrystals released electrochemically from graphite. J Am Chem Soc. 2009;131:4564–5.
Zhu SJ, Song YB, Zhao XH, Shao JR, Zhang JH, Yang B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res. 2015;8:355–81.
Luo PJG, Sahu S, Yang ST, Sonkar SK, Wang JP, Wang HF, et al. Carbon “quantum” dots for optical bioimaging. J Mater Chem B. 2013;1:2116–27.
Sun YP, Zhou B, Lin Y, Wang W, Fernando KA, Pathak P, et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128:7756–7.
Wang X, Cao L, Yang ST, Lu FS, Meziani MJ, Tian LL, et al. Bandgap-like strong fluorescence in functionalized carbon nanoparticles. Angew Chem Int Edit. 2010;49:5310–4.
Dimos K. Carbon Quantum dots: surface passivation and functionalization. Curr Org Chem. 2016;20:682–95.
Li LB, Dong T. Photoluminescence tuning in carbon dots: surface passivation or/and functionalization, heteroatom doping. J Mater Chem C. 2018;6:7944–70.
Li CL, Ou CM, Huang CC, Wu WC, Chen YP, Lin TE, et al. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J Mater Chem B. 2014;2:4564–71.
Roy P, Periasamy AP, Lin CY, Her GM, Chiu WJ, Li CL, et al. Photoluminescent graphene quantum dots for in vivo imaging of apoptotic cells. Nanoscale. 2015;7:2504–10.
Yang ST, Cao L, Luo PGJ, Lu FS, Wang X, Wang HF, et al. Carbon dots for optical imaging in vivo. J Am Chem Soc. 2009;131:11308–9.
Wang CI, Wu WC, Periasamy AP, Chang HT. Electrochemical synthesis of photoluminescent carbon nanodots from glycine for highly sensitive detection of hemoglobin. Green Chem. 2014;16:2509–14.
Vedamalai M, Periasamy AP, Wang CW, Tseng YT, Ho LC, Shih CC, et al. Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells. Nanoscale. 2014;6:13119–25.
Miao P, Han K, Tang YG, Wang BD, Lin T, Cheng WB. Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale. 2015;7:1586–95.
Li HT, Kang ZH, Liu Y, Lee ST. Carbon nanodots: synthesis, properties and applications. J Mater Chem. 2012;22:24230–53.
Hsu PC, Chang HT. Synthesis of high-quality carbon nanodots from hydrophilic compounds: role of functional groups. Chem Commun. 2012;48:3984–6.
Hsu PC, Shih ZY, Lee CH, Chang HT. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012;14:917–20.
Hsu PC, Chen PC, Ou CM, Chang HY, Chang HT. Extremely high inhibition activity of photoluminescent carbon nanodots toward cancer cells. J Mater Chem B. 2013;1:1774–81.
Sahu S, Behera B, Maiti TK, Mohapatra S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun. 2012;48:8835–7.
Zhu LL, Yin YJ, Wang CF, Chen S. Plant leaf-derived fluorescent carbon dots for sensing, patterning and coding. J Mater Chem C. 2013;1:4925–32.
Sabet M, Mahdavi K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl Surf Sci. 2019;463:283–91.
Liu ML, Chen BB, Li CM, Huang CZ. Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019;21:449–71.
Siddique A, Pramanick AK, Chatterjee S, Ray M. Amorphous carbon dots and their remarkable ability to detect 2,4,6-trinitrophenol. Sci Rep UK. 2018;8:9770.
Zhu SJ, Wang L, Zhou N, Zhao XH, Song YB, Maharjan S, et al. The crosslink enhanced emission (cee) in non-conjugated polymer dots: from the photoluminescence mechanism to the cellular uptake mechanism and internalization. Chem Commun. 2014;50:13845–8.
Shi WB, Wang QL, Long YJ, Cheng ZL, Chen SH, Zheng HZ, et al. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun. 2011;47:6695–7.
Long YJ, Wang XL, Shen DJ, Zheng HZ. Detection of glucose based on the peroxidase-like activity of reduced state carbon dots. Talanta. 2016;159:122–6.
Sun HJ, Zhao AD, Gao N, Li K, Ren JS, Qu XG. Deciphering a nanocarbon-based artificial peroxidase: chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew Chem Int Edit. 2015;54:7176–80.
Zhong QM, Chen YY, Qin X, Wang YL, Yuan CL, Xu YJ. Colorimetric enzymatic determination of glucose based on etching of gold nanorods by iodine and using carbon quantum dots as peroxidase mimics. Microchim Acta. 2019;186:161.
Nirala NR, Khandelwal G, Kumar B, Vinita Prakash R, Kumar V. One step electro-oxidative preparation of graphene quantum dots from wood charcoal as a peroxidase mimetic. Talanta. 2017;173:36–43.
Wang H, Liu CQ, Liu Z, Ren JS, Qu XG. Specific oxygenated groups enriched graphene quantum dots as highly efficient enzyme mimics. Small. 2018;14:1703710.
Nirala NR, Abraham S, Kumar V, Bansal A, Srivastava A, Saxena PS. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens Actuat B Chem. 2015;218:42–50.
Lin LP, Song XH, Chen YY, Rong MC, Zhao TT, Wang YR, et al. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal Chim Acta. 2015;869:89–95.
Hu Y, Gao XJ, Zhu Y, Muhammad F, Tan S, Cao W, et al. Nitrogen-doped carbon nanomaterials as highly active and specific peroxidase mimics. Chem Mater. 2018;30:6431–9.
Liu S, Tian JQ, Wang L, Luo YL, Sun XP. A general strategy for the production of photoluminescent carbon nitride dots from organic amines and their application as novel peroxidase-like catalysts for colorimetric detection of H2O2 and glucose. RSC Adv. 2012;2:411–3.
Lin S, Zhang Y, Cao W, Wang X, Qin L, Zhou M, et al. Nucleobase-mediated synthesis of nitrogen-doped carbon nanozymes as efficient peroxidase mimics. Dalton Trans. 2019;8:1993–9.
Shamsipur M, Safavi A, Mohammadpour Z. Indirect colorimetric detection of glutathione based on its radical restoration ability using carbon nanodots as nanozymes. Sensor Actuat B Chem. 2014;199:463–9.
Bano D, Kumar V, Singh VK, Chandra S, Singh DK, Yadav PK, et al. A facile and simple strategy for the synthesis of label free carbon quantum dots from the latex of euphorbia milli and its peroxidase-mimic activity for the naked eye detection of glutathione in a human blood serum. ACS Sustain Chem Eng. 2019;7:1923–32.
Zheng AX, Cong ZX, Wang JR, Li J, Yang HH, Chen GN. Highly-efficient peroxidase-like catalytic activity of graphene dots for biosensing. Biosens Bioelectron. 2013;49:519–24.
Yadav PK, Singh VK, Chandra S, Bano D, Kumar V, Talat M, et al. Green synthesis of fluorescent carbon quantum dots from azadirachta indica leaves and their peroxidase-mimetic activity for the detection of H2O2 and ascorbic acid in common fresh fruits. ACS Biomater Sci Eng. 2019;5:623–32.
Zhu WF, Zhang J, Jiang ZC, Wang WW, Liu XH. High-quality carbon dots: synthesis, peroxidase-like activity and their application in the detection of H2O2, Ag+ and Fe3+. RSC Adv. 2014;4:17387–92.
Mohammadpour Z, Safavi A, Shamsipur M. A new label free colorimetric chemosensor for detection of mercury ion with tunable dynamic range using carbon nanodots as enzyme mimics. Chem Eng J. 2014;255:1–7.
Wang B, Chen YF, Wu YY, Weng B, Liu YS, Li CM. Synthesis of nitrogen- and iron-containing carbon dots, and their application to colorimetric and fluorometric determination of dopamine. Microchim Acta. 2016;183:2491–500.
Cao SS, Kang FF, Li P, Chen RF, Liu H, Wei Y. Photoassisted hetero-fenton degradation mechanism of acid blue 74 by a gamma-Fe2O3 catalyst. RSC Adv. 2015;5:66231–8.
Shete MD, Fernandes JB. A simple one step solid state synthesis of nanocrystalline ferromagnetic alpha-Fe2O3 with high surface area and catalytic activity. Mater Chem Phys. 2015;165:113–8.
Dong YM, Zhang JJ, Jiang PP, Wang GL, Wu XM, Zhao H, et al. Superior peroxidase mimetic activity of carbon dots-Pt nanocomposites relies on synergistic effects. New J Chem. 2015;39:4141–6.
Zheng C, Ke WJ, Yin TX, An XQ. Intrinsic peroxidase-like activity and the catalytic mechanism of gold@carbon dots nanocomposites. RSC Adv. 2016;6:35280–6.
Guo YL, Liu XY, Wang XD, Iqbal A, Yang CD, Liu WS, et al. Carbon dot/nial-layered double hydroxide hybrid material: facile synthesis, intrinsic peroxidase-like catalytic activity and its application. RSC Adv. 2015;5:95495–503.
Hassanzadeh J, Khataee A. Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta. 2018;178:992–1000.
Chen SH, Chi MQ, Yang ZZ, Gao M, Wang C, Lu XF. Carbon dots/Fe3O4 hybrid nanofibers as efficient peroxidase mimics for sensitive detection of h2o2 and ascorbic acid. Inorg Chem Front. 2017;4:1621–7.
Yousefinejad S, Rasti H, Hajebi M, Kowsari M, Sadravi S, Honarasa F. Design of C-dots/Fe3O4 magnetic nanocomposite as an efficient new nanozyme and its application for determination of H2O2 in nanomolar level. Sensor Actuat B Chem. 2017;247:691–6.
Zhang L, Hai X, Xia C, Chen XW, Wang JH. Growth of CuO nanoneedles on graphene quantum dots as peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Sensor Actuat B Chem. 2017;248:374–84.
Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80:1339.
Liu WY, Yang HM, Ma C, Ding YN, Ge SG, Yu JH, et al. Graphene-palladium nanowires based electrochemical sensor using ZnFe2O4-graphene quantum dots as an effective peroxidase mimic. Anal Chim Acta. 2014;852:181–8.
Acknowledgements
We are grateful to the Ministry of Science and Technology (MOST) of Taiwan for providing financial support for this study under contracts 107-2113-M-002-015-MY3, and MOST 107-2113-M-018-005.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
The publisher has retracted this article due to an operational error during the publication process. The authors agree with this retraction.
About this article
Cite this article
Wei, SC., Lin, YW. & Chang, HT. RETRACTED ARTICLE: Carbon Dots as Artificial Peroxidases for Analytical Applications. J. Anal. Test. 3, 191–205 (2019). https://doi.org/10.1007/s41664-019-00107-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-019-00107-x