Abstract
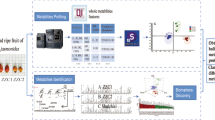
Lettuce (Lactuca sativa L.) is one of the most consumed vegetables in the world and different management practice can result in considerable variability of the secondary metabolites. Flow injection mass spectrometry (FIMS) combined with analysis of variance–principle component analysis (ANOVA–PCA) was used to study differences in the secondary metabolites originating from different lighting conditions (Sunlight, white light, and florescent light) and lettuce varieties (Romaine and Lollo Rossa). Ultra-high-performance liquid chromatography–high-resolution accurate mass spectrometry was used for putative marker compound identification. Quinic acid, caffeic acid, chlorogenic acid, L-chicoric acid, and quercetin malonyl glucoside varied significantly for Romaine lettuce grown under different light conditions. The study showed that the combination of FIMS fingerprinting and ANOVA–PCA can be a useful tool for the characterization of the sources of variance in plant materials regarding to genetic, environmental, and management factors.






Similar content being viewed by others
References
Alarcon-Flores MI, Romero-Gonzalez R, Martinez Vidal JL, Garrido Frenich A. Multiclass determination of phenolic compounds in different varieties of tomato and lettuce by ultra high performance liquid chromatography coupled to tandem mass spectrometry. Int J Food Prop. 2016;19(3):494–507. https://doi.org/10.1080/10942912.2014.978010.
Ribas-Agusti A, Gratacos-Cubarsi M, Sarraga C, Garcia-Regueiro J-A, Castellari M. Analysis of eleven phenolic compounds including novel p-coumaroyl derivatives in lettuce (Lactuca sativa L.) by ultra-high-performance liquid chromatography with photodiode array and mass spectrometry detection. Phytochem Anal. 2011;22(6):555–63. https://doi.org/10.1002/pca.1318.
Viacava GE, Roura SI, Berrueta LA, Iriondo C, Gallo B, Alonso-Salces RM. Characterization of phenolic compounds in green and red oak-leaf lettuce cultivars by UHPLC-DAD-ESI-QToF/MS using MSE scan mode. J Mass Spectrom. 2017;52(12):873–902. https://doi.org/10.1002/jms.4021.
Viacava GE, Roura SI, Lopez-Marquez DM, Berrueta LA, Gallo B, Alonso-Salces RM. Polyphenolic profile of butterhead lettuce cultivar by ultrahigh performance liquid chromatography coupled online to UV-visible spectrophotometry and quadrupole time-of-flight mass spectrometry. Food Chem. 2018;260:239–73.
Kim DI, Pedersen H, Chin CK. Effects of light on berberine production in cell-suspension cultures of Thalictrum-Rugosum. Biotech Lett. 1988;10(10):709–12.
Zhong JJ, Seki T, Kinoshita S, Yoshida T. Effect of light irradiation on anthocyanin production by suspended culture of Perilla-Frutescens. Biotechnol Bioeng. 1991;38(6):653–8. https://doi.org/10.1002/bit.260380610.
Kreuzaler F, Hahlbrock K. Flavonoid glycosides from illuminated cell suspension cultures of Petroselinum-Hortense. Phytochemistry. 1973;12(5):1149–52. https://doi.org/10.1016/0031-9422(73)85031-9.
Wold S, Esbensen K, Geladi P. Principal component analysis. Chemom Intell Lab. 1987;2(1–3):37–52. https://doi.org/10.1016/0169-7439(87)80084-9.
Harrington PDB, Vieira NE, Chen P, Espinoza J, Nien JK, Romero R, et al. Proteomic analysis of amniotic fluids using analysis of variance-principal component analysis and fuzzy rule-building expert systems applied to matrix-assisted laser desorption/ionization mass spectrometry. Chemom Intell Lab Syst. 2006;82(1–2):283–93. https://doi.org/10.1016/j.chemolab.2005.05.011.
Harnly J, Chen P, Sun JH, Huang HL, Colson KL, Yuk J, et al. Comparison of flow injection MS, NMR, and DNA sequencing: methods for identification and authentication of black cohosh (Actaea racemosa). Planta Med. 2016;82(3):250–62. https://doi.org/10.1055/s-0035-1558113.
Chen P, Sun JH, Ford P. Differentiation of the four major species of cinnamons (C-burmannii, C-verum, C-cassia, and C-loureiroi) using a flow injection mass spectrometric (FIMS) fingerprinting method. J Agric Food Chem. 2014;62(12):2516–21. https://doi.org/10.1021/jf405580c.
Huang HL, Sun JH, Mccoy JA, Zhong HY, Fletcher EJ, Harnly J, et al. Use of flow injection mass spectrometric fingerprinting and chemometrics for differentiation of three black cohosh species. Spectrochim Acta B. 2015;105:121–9. https://doi.org/10.1016/j.sab.2014.10.005.
Chen P, Harnly JM, Lester GE. Flow injection mass spectral fingerprints demonstrate chemical differences in rio red grapefruit with respect to year, harvest time, and conventional versus organic farming. J Agric Food Chem. 2010;58(8):4545–53. https://doi.org/10.1021/jf904324c.
Sun JH, Chen P. A flow-injection mass spectrometry fingerprinting method for authentication and quality assessment of Scutellaria lateriflora-based dietary supplements. Anal Bioanal Chem. 2011;401(5):1577–84. https://doi.org/10.1007/s00216-011-5246-2.
Harnly JM, Pastor-Corrales MA, Luthria DL. Variance in the chemical composition of dry beans determined from UV spectral fingerprints. J Agric Food Chem. 2009;57(19):8705–10. https://doi.org/10.1021/jf900852y.
Rajashekar CB, Oh M-M, Carey EE. Organic crop management enhances chicoric acid content in lettuce. Food Nutr Sci. 2012;3(9):1296–302. https://doi.org/10.4236/fns.2012.39171.
Lin L-Z, Harnly J, Zhang R-W, Fan X-E, Chen H-J. Quantitation of the hydroxycinnamic acid derivatives and the glycosides of flavonols and flavones by UV absorbance after identification by LC-MS. J Agric Food Chem. 2012;60(2):544–53. https://doi.org/10.1021/jf204612t.
Abu-Reidah IM, Contreras MM, Arraez-Roman D, Segura-Carretero A, Fernandez-Gutierrez A. Reversed-phase ultra-high-performance liquid chromatography coupled to electrospray ionization-quadrupole-time-of-flight mass spectrometry as a powerful tool for metabolic profiling of vegetables: Lactuca sativa as an example of its application. J Chromatogr A. 2013;1313:212–27. https://doi.org/10.1016/j.chroma.2013.07.020.
Acknowledgements
This research is supported by the Agricultural Research Service of the US Department of Agriculture and an Interagency Agreement with the Office of Dietary Supplements at the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sun, J., Zhang, M., Kubzdela, N. et al. Determination of Variance of Secondary Metabolites in Lettuces Grown Under Different Light Sources by Flow Injection Mass Spectrometric (FIMS) Fingerprinting and ANOVA–PCA. J. Anal. Test. 2, 312–321 (2018). https://doi.org/10.1007/s41664-018-0072-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-018-0072-6