Abstract
Purpose
Laser irradiation (LI) is an emerging antimicrobial strategy that may reduce public health reliance on antibiotics but previous studies reported results based on one wavelength-duration LI, typically on one bacterial species using solid culture. This study aimed to evaluate the effect of increasing duration of 405 nm, 532 nm and 650 nm LI on methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa and Mycobacterium fortuitum in liquid culture.
Methods
Bacterial cultures of 1000 CFU/ml irradiated with 405 nm, 532 nm and 650 nm (200 mW) laser at 1, 2, 5 and 10 min were subcultured on nutrient agar; colony counts pre/post-LI were recorded and survival calculated. Antimicrobial sensitivity and cell morphology pre/post-LI were compared using scanning electron microscopy (SEM) and disc diffusion, respectively.
Results
The 405 nm (blue) LI had the highest bactericidal effect (0–6% survival) on all test bacteria compared to 532 nm (green) (12–49% survival) and 650 nm (red) (33–94% survival) LI. An inverse relationship between LI duration and survival was observed, except in 650 nm LI of P. aeruginosa, whereby survival increased with increasing duration. No change in antibiotic sensitivity was observed for subcultured bacterial colonies post-10 min LI. Although SEM micrographs of bacteria 10 minutes post-405 nm LI showed appreciable cell morphological changes, no significant cellular damage was observed.
Conclusion
These encouraging results confirm bactericidal effects of 405 nm LI on bacteria such as P. aeruginosa and further expand findings on 532 nm and 650 nm LI on bacterial survival, highlighting the potential of LI as novel antimicrobial strategies on opportunistic pathogens such as MRSA, P. aeruginosa and M. fortuitum.

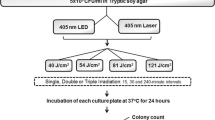
Flowchart of research methodology.




Similar content being viewed by others
References
Dadgostar P (2019) Antimicrobial resistance: implications and costs. Infect Drug Resist 12:3903–3910. https://doi.org/10.2147/IDR.S234610
Theuretzbacher U, Outterson K, Engel A, Karlen A (2019) The global preclinical antibacterial pipeline. Nat Rev Microbiol 18:275–285. https://doi.org/10.1038/s41579-019-0288-0
Hamblin MR, Abrahamse H (2019) Can light-based approaches overcome antimicrobial resistance? Drug Dev Res 80(1):48–67. https://doi.org/10.1002/ddr.21453
Fu XJ, Fang Y, Yao M (2013) Antimicrobial photodynamic therapy for methicillin-resistant Staphylococcus aureus infection. Biomed Res Int 2013:159157–159159. https://doi.org/10.1155/2013/159157
Akram FE, El-Tayeb T, Abou-Aisha K, El-Azizi M (2016) A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). Ann Clin Microbiol Antimicrob 15(1):48. https://doi.org/10.1186/s12941-016-0164-y
Azadgoli B, Baker RY (2016) Laser applications in surgery. Ann Transl Med 4(23):452. https://doi.org/10.21037/atm.2016.11.51
Greco G, Di Piazza S, Chan J, Zotti M, Hanna R, Gheno E, Zekiy AO, Pasquale C, De Angelis N, Amaroli A (2019) Newly formulated 5% 5-aminolevulinic acid phototherapy on Candida albicans. Photodiagn Photodyn Ther 29:101575. https://doi.org/10.1016/j.pdpdt.2019.10.010
Azizi A, Shohrati P, Goudarzi M, Lawaf S, Rahimi A (2019) Comparison of the effect of photodynamic therapy with curcumin and methylene Blue on streptococcus mutans bacterial colonies. Photodiagn Photodyn Ther 27:203–209. https://doi.org/10.1016/j.pdpdt.2019.06.002
Terada C, Imamura T, Ohshima T, Maeda N, Tatehara S, Tokuyama-Toda R, Yamachika S, Toyoda N, Satomura K (2018) The effect of irradiation with a 405 nm blue-violet laser on the bacterial adhesion on the osteosynthetic biomaterials. Int J Photoenergy 2018:1–10. https://doi.org/10.1155/2018/2635964
Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK (2008) Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med 40(10):734–737. https://doi.org/10.1002/lsm.20724
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, Aboderin AO, al-Abri SS, Awang Jalil N, Benzonana N, Bhattacharya S, Brink AJ, Burkert FR, Cars O, Cornaglia G, Dyar OJ, Friedrich AW, Gales AC, Gandra S, Giske CG, Goff DA, Goossens H, Gottlieb T, Guzman Blanco M, Hryniewicz W, Kattula D, Jinks T, Kanj SS, Kerr L, Kieny MP, Kim YS, Kozlov RS, Labarca J, Laxminarayan R, Leder K, Leibovici L, Levy-Hara G, Littman J, Malhotra-Kumar S, Manchanda V, Moja L, Ndoye B, Pan A, Paterson DL, Paul M, Qiu H, Ramon-Pardo P, Rodríguez-Baño J, Sanguinetti M, Sengupta S, Sharland M, Si-Mehand M, Silver LL, Song W, Steinbakk M, Thomsen J, Thwaites GE, van der Meer JWM, van Kinh N, Vega S, Villegas MV, Wechsler-Fördös A, Wertheim HFL, Wesangula E, Woodford N, Yilmaz FO, Zorzet A (2018) Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18(3):318–327. https://doi.org/10.1016/S1473-3099(17)30753-3
Zheng HW, He GX, Song YY, Zhao YL (2017) Antimicrobial susceptibility testing and molecular characterization of Mycobacterium fortuitum isolates in China. Biomed Environ Sci 30(5):376–379
Guffey JS, Wilborn J (2006) In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg 24(6):684–688. https://doi.org/10.1089/pho.2006.24.684
Abana CM, Brannon JR, Ebbott RA, Dunigan TL, Guckes KR, Fuseini H, Powers J, Rogers BR, Hadjifrangiskou M (2017) Characterization of blue light irradiation effects on pathogenic and nonpathogenic Escherichia coli. Microbiologyopen 6(4):e00466. https://doi.org/10.1002/mbo3.466
Ghorbani J, Rahban D, Aghamiri S, Teymouri A, Bahador A (2018) Photosensitizers in antibacterial photodynamic therapy: an overview. Laser Ther 27(4):293–302. https://doi.org/10.5978/islsm.27_18-RA-01
Lan M, Zhao S, Liu W, Lee CS, Zhang W, Wang P (2019) Photosensitizers for photodynamic therapy. Adv Healthc Mater 8(13):e1900132. https://doi.org/10.1002/adhm.201900132
Santos DR, Lourenco MC, Coelho FS, Mello FC, Duarte RS (2016) Resistance profile of strains of Mycobacterium fortuitum isolated from clinical specimens. J Bras Pneumol 42(4):299–301. https://doi.org/10.1590/S1806-37562016000000073
LaBauve AE, Wargo MJ (2012) Growth and laboratory maintenance of Pseudomonas aeruginosa. Curr Protoc Microbiol Chapter 6:Unit 6E 1. doi:https://doi.org/10.1002/9780471729259.mc06e01s25
McBirney SE, Trinh K, Wong-Beringer A, Armani AM (2016) Wavelength-normalized spectroscopic analysis of Staphylococcus aureus and Pseudomonas aeruginosa growth rates. Biomed Opt Express 7(10):4034–4042. https://doi.org/10.1364/BOE.7.004034
Cheesbrough M (2006) District laboratory practice in tropical countries, vol 2, 2nd edn. Cambridge University Press, New York
CLSI (2018) Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, Wayne
CLSI (2018) Laboratory detection and identification of mycobacteria. Clinical and Laboratory Standards Institute, Wayne
Li G, Lian LL, Wan L, Zhang J, Zhao X, Jiang Y, Zhao LL, Liu H, Wan K (2013) Antimicrobial susceptibility of standard strains of nontuberculous mycobacteria by microplate Alamar Blue assay. PLoS One 8(12):e84065. https://doi.org/10.1371/journal.pone.0084065
Missiakas DM, Schneewind O (2013) Growth and laboratory maintenance of Staphylococcus aureus. Curr Protoc Microbiol Chapter 9:Unit 9C 1. doi:https://doi.org/10.1002/9780471729259.mc09c01s28
Dominguez MC, de La Rosa M, Borobio MV (2001) Application of a spectrophotometric method for the determination of post-antibiotic effect and comparison with viable counts in agar. J Antimicrob Chemother 47(4):391–398. https://doi.org/10.1093/jac/47.4.391
Murtey MD, Ramasamy P (2016) Sample preparations for scanning electron microscopy-life sciences. In: Janecek MKR (ed) Modern electron microscopy in physical and life sciences. Intech Open. https://doi.org/10.5772/61720
Kim S, Kim J, Lim W, Jeon S, Kim O, Koh JT, Kim CS, Choi HR, Kim OJ (2013) In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomed Laser Surg 31(11):554–562. https://doi.org/10.1089/pho.2012.3343
Grinholc M, Rodziewicz A, Forys K, Rapacka-Zdonczyk A, Kawiak A, Domachowska A, Golunski G, Wolz C, Mesak L, Becker K, Bielawski KP (2015) Fine-tuning recA expression in Staphylococcus aureus for antimicrobial photoinactivation: importance of photo-induced DNA damage in the photoinactivation mechanism. Appl Microbiol Biotechnol 99(21):9161–9176. https://doi.org/10.1007/s00253-015-6863-z
Yuan X, Song Y, Song Y, Xu J, Wu Y, Glidle A, Cusack M, Ijaz UZ, Cooper JM, Huang WE, Yin H (2018) Effect of laser irradiation on cell function and its implications in Raman spectroscopy. Appl Environ Microbiol 84(8):e02508–e02517. https://doi.org/10.1128/AEM.02508-17
Plavskii VY, Mikulich AV, Tretyakova AI, Leusenka IA, Plavskaya LG, Kazyuchits OA, Dobysh II, Krasnenkova TP (2018) Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J Photochem Photobiol B 183:172–183. https://doi.org/10.1016/j.jphotobiol.2018.04.021
Gwynne PG, M P (2018) Light as a broad-spectrum antimicrobial. Front Microb 9:1–9
Lubart R, Lipovski A, Nitzan Y, Friedmann H (2011) A possible mechanism for the bactericidal effect of visible light. Laser Ther 20(1):17–22. https://doi.org/10.5978/islsm.20.17
Alvarez MG, Milanesio M, Rivarola V, Durantini E, Batlle A, Fukuda H (2009) Endogenous and exogenous porphyrins as photosensitizers in the Hep-2 human carcinoma cell line. Cell Mol Biol (Noisy-le-grand) 55(2):8–14
Alvarez MG, Principe F, Milanesio ME, Durantini EN, Rivarola V (2005) Photodynamic damages induced by a monocationic porphyrin derivative in a human carcinoma cell line. Int J Biochem Cell Biol 37(12):2504–2512. https://doi.org/10.1016/j.biocel.2005.06.016
Ramakrishnan P, Maclean M, MacGregor SJ, Anderson JG, Grant MH (2014) Differential sensitivity of osteoblasts and bacterial pathogens to 405-nm light highlighting potential for decontamination applications in orthopedic surgery. J Biomed Opt 19(10):105001. https://doi.org/10.1117/1.JBO.19.10.105001
Antoniou C, Dessinioti C, Sotiriadis D, Kalokasidis K, Kontochristopoulos G, Petridis A, Rigopoulos D, Vezina D, Nikolis A (2016) A multicenter, randomized, split-face clinical trial evaluating the efficacy and safety of chromophore gel-assisted blue light phototherapy for the treatment of acne. Int J Dermatol 55(12):1321–1328. https://doi.org/10.1111/ijd.13349
Matias VR, Al-Amoudi A, Dubochet J, Beveridge TJ (2003) Cryo-transmission electron microscopy of frozen-hydrated sections of Escherichia coli and Pseudomonas aeruginosa. J Bacteriol 185(20):6112–6118. https://doi.org/10.1128/jb.185.20.6112-6118.2003
Cazares-Dominguez V, Cruz-Cordova A, Ochoa SA, Escalona G, Arellano-Galindo J, Rodriguez-Leviz A et al (2015) Vancomycin tolerant, methicillin-resistant Staphylococcus aureus reveals the effects of vancomycin on cell wall thickening. PLoS One 10(3):e0118791. https://doi.org/10.1371/journal.pone.0118791
Mai-Prochnow A, Clauson M, Hong J, Murphy AB (2016) Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep 6:38610. https://doi.org/10.1038/srep38610
Velayati AA, Farnia P, Ibrahim TA, Haroun RZ, Kuan HO, Ghanavi J, Farnia P, Kabarei AN, Tabarsi P, Omar AR, Varahram M, Masjedi MR (2009) Differences in cell wall thickness between resistant and nonresistant strains of Mycobacterium tuberculosis: using transmission electron microscopy. Chemotherapy 55(5):303–307. https://doi.org/10.1159/000226425
Aires de Sousa M, de Lencastre H (2003) Evolution of sporadic isolates of methicillin-resistant Staphylococcus aureus (MRSA) in hospitals and their similarities to isolates of community-acquired MRSA. J Clin Microbiol 41(8):3806–3815. https://doi.org/10.1128/jcm.41.8.3806-3815.2003
Menendez Mdel C, Rebollo MJ, Nunez Mdel C, Cox RA, Garcia MJ (2005) Analysis of the precursor rRNA fractions of rapidly growing mycobacteria: quantification by methods that include the use of a promoter (rrnA P1) as a novel standard. J Bacteriol 187(2):534–543. https://doi.org/10.1128/JB.187.2.534-543.2005
Li J, Xie S, Ahmed S, Wang F, Gu Y, Zhang C, Chai X, Wu Y, Cai J, Cheng G (2017) Antimicrobial activity and resistance: influencing factors. Front Pharmacol 8:364. https://doi.org/10.3389/fphar.2017.00364
Pontes MH, Groisman EA (2019) Slow growth determines nonheritable antibiotic resistance in Salmonella enterica. Sci Signal 12(592):eaax3938. https://doi.org/10.1126/scisignal.aax3938
Che Hamzah AM, Yeo CC, Puah SM, Chua KH, Chew CH (2019) Staphylococcus aureus infections in Malaysia: a review of antimicrobial resistance and characteristics of the clinical isolates, 1990-2017. Antibiotics (Basel) 8(3). https://doi.org/10.3390/antibiotics8030128
Tummler B (2019) Emerging therapies against infections with Pseudomonas aeruginosa. F1000Res 8. doi:https://doi.org/10.12688/f1000research.19509.1
Acknowledgments
The authors would like to acknowledge Ghows Azzam, Mohd Nor Norazmi, Naser Mahmoud Ahmad for their support and critical input.
Funding
This study was funded by the Ministry of Education Malaysia LRGS (203.PPSK.67212002) and FRGS (203.PBIOLOGI.6711581).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Shammary, A.A.K., Mohd Ma’amor, N.A.A., Chen, S.Q. et al. Bactericidal effects of in vitro 405 nm, 530 nm and 650 nm laser irradiation on methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa and Mycobacterium fortuitum. Laser Dent Sci 4, 111–121 (2020). https://doi.org/10.1007/s41547-020-00097-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-020-00097-5