Abstract
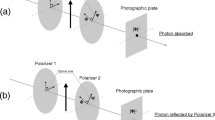
Consciousness and reality are related through the “measurement problem” in quantum mechanics, i.e., why we do not consciously perceive particles as quantum superpositions of multiple possibilities, as they appear to be when unobserved, but rather perceive them consciously as being in definite states or locations. Quantum pioneers Niels Bohr, John von Neumann, Eugene Wigner, and Henry Stapp concluded that subjective conscious observation causes quantum state reduction (“subjective reduction” (SR)), that “consciousness collapses the wavefunction.” However, Sir Roger Penrose suggested instead that quantum state reduction occurs spontaneously due to an objective threshold property (“objective reduction” (OR)) in fundamental spacetime geometry, collapsing the wave function and causing moments of conscious experience (“collapse causes consciousness,” or “collapse is consciousness”). Penrose OR would be occurring ubiquitously and randomly in the environment (“decoherence”) resulting in ubiquitous proto-conscious moments. The Penrose–Hameroff “Orch OR” model of orchestrated objective reduction suggests that microtubules inside brain neurons “orchestrate” quantum computations which “halt” by Orch OR to produce moments of a full, rich conscious experience.






Similar content being viewed by others
References
Craddock, T.J.A., St George, M., Freedman, H., Barakat, K.H., Damaraju, S., Hameroff, S., Tuszynski, J.A. (2012). Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: Implications for side effects of general anesthesia. PLoS One 7: e37251.
Craddock, T. J. A., Hameroff, S. R., Ayoub, A. T., Klobukowski, M., & Tuszynski, J. A. (2015). Anesthetics act in quantum channels in brain microtubules to prevent consciousness. Current Topics in Medicinal Chemistry, 15, 523–533.
Craddock, T.J.A., Kurian, P., Preto, J., Sahu, K., Hameroff, S.R., Klobukowski, M., Tuszynski, J.A. (2017). Anesthetic Alterations of Collective Terahertz Oscillations in Tubulin Correlate with Clinical Potency: Implications for Anesthetic Action and Post-Operative Cognitive Dysfunction. Scientific Reports, 7, 9877.
Dennis, E., Preskill, J. (2002). Topological quantum memory. Journal of Mathematical Physics, 43, 4452.
Emerson, D., Weiser, B., Psonis, J., Liao, Z., Taratula, O., Fiamengo, A., et al. (2013). Direct modulation of microtubule stability contributes to anthracene general anesthesia. Journal of the American Chemical Society, 135(14), 5398.
Everett, H., (1955). Relative state formulation of quantum mechanics. Reviews of Modern Physics 29, 454–462.
Fröhlich, H.(1968). Long range coherence and energy storage in biological systems. International Journal of Quantum Chemistry, 2, 641–649.
Fröhlich, H. (1970). Long range coherence and the actions of enzymes. Nature, 228, 1093.
Fröhlich, H. (1975). The extraordinary dielectric properties of biological materials and the action of enzymes. Proceedings of the National Academy of Sciences of the United States of America, 72, 4211–4215.
Hagan, S., Hameroff, S., & Tuszynski, J. (2001). Quantum computation in brain microtubules? Decoherence and biological feasibility. Physical Review E, 65, 061901.
Hameroff, S. (1998a). Quantum computation in brain microtubules? The Penrose–Hameroff “Orch OR” model of consciousness. Philosophical Transactions of the Royal Society of London. Series A: Mathematical Physical and Engineering Sciences, 356(1998), 1869–1896.
Hameroff, S. (1998b). Anesthesia, consciousness and hydrophobic pockets – A unitary quantum hypothesis of anesthetic action. Toxicology Letters, 100(101), 31–39.
Hameroff, S., & Penrose, R. (1996a). Orchestrated reduction of quantum coherence in brain microtubules: a model for consciousness. Mathematics and Computers in Simulation, 40, 453–480.
Hameroff, S., & Penrose, R. (1996b). Conscious events as orchestrated space–time selections. Journal of Consciousness Studies, 3(1), 36–53.
Hameroff, S., & Penrose, R. (2014). Consciousness in the universe: A review of the ‘Orch OR’theory. Physics of Life Reviews, 11, 39–78.
Hameroff, S, & Penrose, R. (2016). Consciousness in the universe: A review of the ‘Orch OR’theory, In: Biophysics of Consciousness: A Foundational Approach Eds: RR Poznanski, JA Tuszynski, TE Feinberg, World Scientific, Singapore.
Hameroff, S., & Watt, R. (1983). Do anesthetics act by altering electron mobility? Anesthesia and Analgesia, 62, 936–940.
Hameroff, S., Nip, A., Porter, M., & Tuszynski, J. (2002). Conduction pathways in microtubules, biological quantum computation, and consciousness. Biosystems, 64, 149–168.
Hameroff, S. R. (1998). Funda-mentality’: is the conscious mind subtly linked to a basic level of the universe? Trends in Cognitive Sciences, 2, 119–127.
Hameroff, S. R., & Watt, R. C. (1982). Information processing in microtubules. Journal of Theoretical Biology, 98, 549–561.
Ghirardi, G.C., Rimini, A., Weber, T. (1986). Unified dynamics for microscopic and macroscopic systems. Physical Review D, 34, 470.
Koch, C., (2012). Consciousness: Confessions of a Romantic Reductionist, MIT Press, Cambridge MA.
Pan, J. Z., Xi, J., Tobias, J. W., Eckenhoff, M. F., & Eckenhoff, R. G. (2007). Halothane binding proteome in human brain cortex. Journal of Proteome Research, 6, 582–592.
Pan, J. Z., Xi, J., Eckenhoff, M. F., & Eckenhoff, R. G. (2008). Inhaled anesthetics elicit region-specific changes in protein expression in mammalian brain. Proteomics, 8, 2983–2992.
Penrose, R. (1989). The emperor’s new mind: Concerning computers, minds, and the laws of physics. Oxford: Oxford University Press.
Penrose, R. (1994). Shadows of the mind: An approach to the missing science of consciousness. Oxford: Oxford University Press.
Penrose, R. (1996). On gravity’s role in quantum state reduction. General Relativity and Gravitation, 28, 581–600.
Penrose, R. (2004). The road to reality: a complete guide to the laws of the universe Jonathan Cape, London.
Penrose, R., & Hameroff, S. (1995). What gaps? Reply to Grush and Churchland. Journal of Consciousness Studies, 2, 98–112.
Rasmussen, S., Karampurwala, H., Vaidyanath, R., Jensen, K., & Hameroff, S. (1990). Computational connectionism within neurons: A model of cytoskeletal automata subserving neural networks. Physica D: Nonlinear Phenomena, 42, 428–449.
Sahu, S., Ghosh, S., Ghosh, B., Aswani, K., Hirata, K., Fujita, D., et al. (2013a). Atomic water channel controlling remarkable properties of a single brain microtubule: Correlating single protein to its supramolecular assembly. Biosensors & Bioelectronics, 47, 141–148.
Sahu, S., Ghosh, S., Hirata, K., Fujita, D., & Bandyopadhyay, A. (2013b). Multi-level memory-switching properties of a single brain microtubule. Applied Physics Letters, 102, 123701.
Sahu, S., Ghosh, S., Fujita, D., Bandyopadhyay, A. (2014). Live visualizations of single isolated tubulin protein self-assembly via tunneling current: effect of electromagnetic pumping during spontaneous growth of microtubule, Scientific Reports, 4, 7303.
Sataric, M.V., Zekovic, S., Tuszynski, J.A., Pokorny, J. (1998). The Mossbauer effect as a possible tool in detecting nonlinear excitations in microtubules. Physical Review E, 58, 6333–6339.
Smith, S., Watt, R., & Hameroff, S. (1984). Cellular automata in cytoskeletal lattice proteins. Physica D, 10, l68–l74.
Stapp, H.P.. (1993) Mind, matter and quantum mechanics, Springer-Verlag, Berlin, Heidelberg.
Stapp, H.P.. (2007) Mindful universe: Quantum mechanics and the participating observer. Springer.
Tegmark, M. (2000). The importance of quantum decoherence in brain processes. Physical Review E, 61, 4194–4206.
Tononi, G. (2012). Phi: A voyage from the brain to the soul Pantheon Books, New York.
Whitehead, A. N. (1929). Process and reality. New York (NY): MacMillan.
Whitehead, A. N. (1933). Adventure of ideas. London: MacMillan.
Wigner, E.P. (1961). Remarks on the mind body question, In: J.A. Wheeler, W.H. Zurek (Eds.) Quantum theory and measurement, Princeton University Press, Princeton.
Xi, J., Liu, R., Asbury, G. R., Eckenhoff, M. F., & Eckenhoff, R. G. (2004). Inhalational anesthetic-binding proteins in rat neuronal membranes. Journal of Biological Chemistry, 279, 19628–19633.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hameroff, S. Consciousness and Quantum State Reduction—Which Comes First?. Act Nerv Super 61, 31–40 (2019). https://doi.org/10.1007/s41470-019-00053-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41470-019-00053-0