Abstract
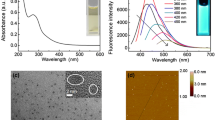
Mercury ions have been considered highly toxic to human health. What would be great is to develop the ionic probes without any toxicities themselves. Here, we report a friendly, highly sensitive mercury (II) ionic probe, water-soluble photoluminescence carbon dots which were synthesized by simply hydrothermal treatment of fresh cherry tomatoes without adding any other reagents. The ultra-small (<1 nm) carbon dots show robust excitation-depended photoluminescence under a wide pH range (4–10) or a strong ionic strength of up to 1 M, and the detection limit of mercury (II) has been determined as low as 18 nM. We envision such water-soluble, biocompatible carbon dots that could be applied to biolabeling, bio-imaging, and biosensing fields.



Similar content being viewed by others
References
W. Zheng, M. Aschner, J.F. Ghersi-Egea, Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol. 192, 1–11 (2003). doi:10.1016/s0041-008x(03)00251-5
Agency U. E. P., Regulatory Impact Analysis of the Clean Air Mercury Rule EPA-452/R-05 003 (Research Triangle Park, North Carolina, 2005)
Y.Q. Wang, Y. Liu, X.W. He et al., Highly sensitive synchronous fluorescence determination of mercury (II) based on the denatured ovalbumin coated CdTe QDs. Talanta 99, 69–74 (2012). doi:10.1016/j.talanta.2012.04.064
M.R. Chao, Y.Z. Chang, J.L. Chen, Hydrophilic ionic liquid-passivated CdTe quantum dots for mercury ion detection. Biosens. Bioelectron. 42, 397–402 (2013). doi:10.1016/j.bios.2012.10.065
J. Ke, X. Li, Q. Zhao et al., Ultrasensitive quantum dot fluorescence quenching assay for selective detection of mercury ions in drinking water. Sci. Rep. 4, 5624 (2014). doi:10.1038/srep05624
J. Pei, H. Zhu, X. Wang et al., Synthesis of cysteamine-coated CdTe quantum dots and its application in mercury (II) detection. Anal. Chim. Acta 757, 63–68 (2012). doi:10.1016/j.aca.2012.10.037
M. Bottini, T. Mustelin, Carbon materials—nanosynthesis by candlelight. Nat. Nanotechnol. 2, 599–600 (2007). doi:10.1038/nnano.2007.316
A.P. Demchenko, M.O. Dekaliuk, Novel fluorescent carbonic nanomaterials for sensing and imaging. Methods Appl. Fluoresc. 1, 1–17 (2013). doi:10.1088/2050-6120/1/4/042001
H.T. Li, Z.H. Kang, Y. Liu et al., Carbon nanodots: synthesis, properties and applications. J. Mater. Chem. 22, 24230–24253 (2012). doi:10.1039/C2jm34690g
W. Lu, X. Qin, S. Liu et al., Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal. Chem. 84, 5351–5357 (2012). doi:10.1021/ac3007939
C. Zhu, J. Zhai, S. Dong, Bifunctional fluorescent carbon nanodots: green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Commun. 48, 9367–9369 (2012). doi:10.1039/c2cc33844k
J.J. Zhou, Z.H. Sheng, H.Y. Han et al., Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 66, 222–224 (2012). doi:10.1016/j.matlet.2011.08.081
Y.X. Sun, Z.W. He, X.B. Sun et al., Synthesis of water-soluble fluorescent carbon dots from a one-step hydrothermal method with potato. Adv. Mater. Res. 873, 770–776 (2013). doi:10.4028/www.scientific.net/AMR.873.770
B. Yin, J. Deng, X. Peng et al., Green synthesis of carbon dots with down- and up-conversion fluorescent properties for sensitive detection of hypochlorite with a dual-readout assay. Analyst 138, 6551–6557 (2013). doi:10.1039/c3an01003a
L. Cao, X. Wang, M.J. Meziani et al., Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 129, 11318–11319 (2007). doi:10.1021/Ja073527l
C.M. Yu, X.Z. Li, F. Zeng et al., Carbon-dot-based ratiometric fluorescent sensor for detecting hydrogen sulfide in aqueous media and inside live cells. Chem. Commun. 49, 403–405 (2013). doi:10.1039/C2cc37329g
W. Wang, Y. Li, L. Cheng et al., Water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J. Mater. Chem. B 2, 46–48 (2014). doi:10.1039/c3tb21370f
S.N. Qu, X.Y. Wang, Q.P. Lu et al., A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew. Chem. Int. Edit. 51, 12215–12218 (2012). doi:10.1002/anie.201206791
W.F. Zhang, H. Zhu, S.F. Yu et al., Observation of lasing emission from carbon nanodots in organic solvents. Adv. Mater. 24, 2263–2267 (2012). doi:10.1002/adma.201104950
Y. Sha, J. Lou, S. Bai et al., Hydrothermal synthesis of nitrogen-containing carbon nanodots as the high-efficient sensor for copper(II) ions. Mater. Res. Bull. 48, 1728–1731 (2013). doi:10.1016/j.materresbull.2012.12.010
S. Pei, J. Zhang, M. Gao et al., A facile hydrothermal approach towards photoluminescent carbon dots from amino acids. J. Colloid Interface Sci. 439, 129–133 (2015). doi:10.1016/j.jcis.2014.10.030
M. Yuan, R. Zhong, H. Gao et al., One-step, green and economic synthesis of water-soluble photoluminescent carbon dots by hydrothermal treatment of wheat straw and their bio-applications in labeling, imaging and sensing. Appl. Surf. Sci. 355, 1136–1144 (2015). doi:10.1016/j.apsusc.2015.07.095
D.Y. Pan, J.C. Zhang, Z. Li et al., Observation of pH-, solvent-, spin-, and excitation-dependent blue photoluminescence from carbon nanoparticles. Chem. Commun. 46, 3681–3683 (2010). doi:10.1039/C000114g
D.Y. Pan, J.C. Zhang, Z. Li et al., Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 22, 734–738 (2010). doi:10.1002/adma.200902825
Y.M. Long, C.H. Zhou, Z.L. Zhang et al., Shifting and non-shifting fluorescence emitted by carbon nanodots. J. Mater. Chem. 22, 5917–5920 (2012). doi:10.1039/C2jm30639e
L.B. Tang, R.B. Ji, X.K. Cao et al., Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 6, 5102–5110 (2012). doi:10.1021/Nn300760g
F. Zhang, Z. Ali, F. Amin et al., In vitro and intracellular sensing by using the photoluminescence of quantum dots. Anal. Bioanal. Chem. 397, 935–942 (2010). doi:10.1007/s00216-010-3609-8
L. Zhou, Y. Lin, Z. Huang et al., Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem. Commun. 48, 1147–1149 (2012). doi:10.1039/c2cc16791c
R.B. Zhong, Y.S. Liu, P. Zhang et al., Discrete nanoparticle-BSA conjugates manipulated by hydrophobic interaction. Acs Appl. Mater. Interfaces 6, 19465–19470 (2014). doi:10.1021/Am506497s
M. Yuan, R. Zhong, X. Yun et al., A fluorimetric study on the interaction between a Trp-containing beta-strand peptide and amphiphilic polymer-coated gold nanoparticles. Luminescence (2015). doi:10.1002/bio.2920
Y. Liu, P. Zhang, R. Zhong et al., Fluorimetric study on the interaction between fluoresceinamine and bovine serum albumin. Nucl. Sci. Tech. 26, 030505 (2015). doi:10.13538/j.1001-8042/nst.26.030505
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (Nos. 21171086 and 81160213), and Inner Mongolia Grassland Talent (No. 108-108038), Natural Science Foundation of Inner Mongolia Autonomous Region of China (Nos. 2013MS1121 and 2015MS0806), Inner Mongolia Department of Science and Technology (No. 211-202077), and the Inner Mongolia Agricultural University (Nos. 109-108040, 211-109003, and 211-206038).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, P., Zhong, RB., Yuan, M. et al. Mercury (II) detection by water-soluble photoluminescent ultra-small carbon dots synthesized from cherry tomatoes. NUCL SCI TECH 27, 35 (2016). https://doi.org/10.1007/s41365-016-0038-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-016-0038-1