Abstract
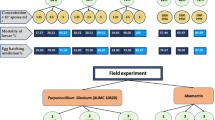
Fluazaindolizine (Reklemel™ active) is a novel sulfonamide and active ingredient (a.i) of the nematicide Salibro™. Its bio-compatibility with Pasteuria penetrans (Pp), a bacterial parasite of root-knot nematodes (Meloidogyne spp.) has been proven in a previous study, where the number of attached spores on juveniles was not affected when spores were incubated in the nematicide, at concentrations of 5–250 ppm a.i for a period of 1–21 days. In the current follow-up study, spores of a single Pp isolate (Pp3) or a blend (Ppblend) of six isolates, were incubated in fluazaindolizine [Salibro™ 500SC, 50% (a.i) at 250 ppm a.i.] for 24 or 26 days. The suspensions were washed through a cellulose filter (1 µm) five times to remove the nematicide while collecting the spores on the filter surface. Juveniles of M. javanica, M. incognita and M. hapla were exposed to these spore suspensions in Petri dishes and the number of attached spores recorded after 24 h. The number of attached spores which were incubated in the nematicide did not differ or was even significantly higher, to that of untreated spores, incubated in water. The spore encumbered juveniles were used to infect tomatoes or peppers which were uprooted and checked after 50 days. The pre-exposure to fluazaindolizine did not alter the ability of the spores to infect females and prevent egg laying. Furthermore, it was demonstrated that the fluazaindolizine-treated spores produced new spores inside the infected females of M. javanica that readily attached to juveniles like these derived from untreated spores.
Similar content being viewed by others
References
Becker JO, Ploeg A, Nuñez JJ (2019) Multi-year field evaluation of fluorinated nematicides against Meloidogyne incognita in carrots. Plant Dis 103:2392–2396. https://doi.org/10.1094/PDIS-03-19-0489-RE
Brown SM, Nordmeyder D (1985) Synergistic reduction in root galling by Meloidogyne javanica with Pasteuria penetrans and nematicides. Revue De Nematologie 8:285–286
Chen ZX, Dickson DW (1998) Review of Pasteuria penetrans: biology, ecology and biological control potential. J Nematol 30:313–340
Davies KG, Kerry BR, Flynn CA (1988) Observations on the pathogenicity of Pasteuria penetrans, a parasite of root-knot nematodes. Annals of Applied Biology 112:491–501
Davies KG, Laird V, Kerry BR (1991) The motility, development and infection of Meloidogyne incognita encumbered with spores of the obligate hyperparasite Pasteuria penetrans. Revue De Nematologie 14:611–618
Gonçalves AR, Conceição IL, Kormpi M, Tzortzakakis EA (2020) Lavandula angustifolia and Oxalis pes-caprae, hosts of Meloidogyne hapla and Meloidogyne javanica-a note for Meloidgyne luci in Greece. Hellenic Plant Prot J 13:78–82. https://doi.org/10.2478/hppj-2020-0008
Hague NGM, Gowen SR (1987) Chemical control of nematodes. In: Brown RH, Kerry BR (eds) Principle and practice of nematode control in crops. Academic Press, Australia, pp 131–178
Hajihassani A, Richard F, Davis RF, Timper P (2019) Evaluation of selected nonfumigant nematicides on increasing inoculation densities of Meloidogyne incognita on cucumber. Plant Dis 103:3161–3165. https://doi.org/10.1094/PDIS-04-19-0836-RE
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter 57:1025–1028
Kokalis-Burelle N (2015) Pasteuria penetrans for Control of Meloidogyne incognita on Tomato and Cucumber, and M. arenaria on Snapdragon. J Nematol 47(3): 207–213.
Mankau R, Prasad N (1972) Possibilities and problems in the use of a sporozoan endoparasite for biological control of plant parasitic nematodes. Nematropica 2:7–8
Mankau R, Prasad N (1977) Infectivity of Bacillus penetrans in plant parasitic nematodes. J Nematol 9:40–45
Nasiou E, Thoden T, Pardavella IV, Tzortzakakis EA (2020) Compatibility of fluazaindolizine and oxamyl with Pasteuria penetrans on spore attachment to juveniles of Meloidogyne javanica and M. incognita. J Nematol. https://doi.org/10.21307/jofnem-2020-070.
Nishizawa T (1989) Comparison of heat resistance and nematicide resistance of endospores of Pasteuria penetrans from Meloidogyne incognita with a related bacterium parasitizing Heterodera glycines. J Nematol 21:576–577
Phani V, Rao U (2018) Revisiting the life-cycle of Pasteuria penetrans infecting Meloidogyne incognita under soil-less medium, and effect of Streptomycin Sulfate on its Development. J Nematol 50: 91–98. https://doi.org/10.21307/jofnem-2018-022
Qiao K, Liua Q, Zhang S (2021) Evaluation of fluazaindolizine, a new nematicide for management of Meloidogyne incognita in squash in calcareous soils. Crop Protection 143. https://doi.org/10.1016/j.cropro.2020.105469
Sayre RM, Starr MP (1985) Pasteuria penetrans (ex Thorne, 1940) nom. Rev., comb. N., sp.n., a mycelial and endospore-forming bacterium parasitic in plant–parasitic nematodes. Proc Helmintol Soc Washington 52:149–165
Spaull VW (1984) Observations on Bacillus penetrans infecting Meloidogyne in sugarcane fields in South Africa. Revue De Nematol 7:277–282
Stirling GR, Wachtel MF (1980) Mass production of Bacillus penetrans for the biological control of root-knot nematodes. Nematologica 26:308–312
Stirling GR, Sharma RD, Perry J (1990) Attachment of Pasteuria penetrans spores to the root-knot nematode Meloidogyne javanica in soil and its effects on infectivity. Nematologica 36:245–252
Talavera M, Thoden TC, Vela-Delgado MD, Verdejo-Lucas S, Sánchez-Moreno S (2021) The impact of fluazaindolizine on free-living nematodes and the nematode community structure in a root-knot nematode infested vegetable production system. Pest Manag Sci 77:5220–5227. https://doi.org/10.1002/ps.6563
Thoden T, Pardavella IV, Tzortzakakis EA (2019) In vitro sensitivity of different populations of Meloidogyne javanica and M. incognita to the nematicides Salibro™ and Vydate®. Nematology 21:889–893
Thoden TC, Wiles JA (2019) Biological attributes of Salibro ™, a novel sulfonamide nematicide. Part 1: Impact on the fitness of Meloidogyne incognita. M. hapla and Acrobeloides buetschii. Nematology 21:635–639
Thoden TC, Alkader MA, Markakis EA, Yum MY, Wiles JA (2022) Biological attributes of Salibro™, a novel sulfonamide nematicide. Part 3: biocompatibility with beneficial soil fungi. Nematology 24:915–924. https://doi.org/10.1163/15685411-bja10179
Timper P (2009) Population dynamics of Meloidogyne arenaria and Pasteuria penetrans in a Long-Term Crop Rotation Study. J Nematol 41(4):291–299
Tzortzakakis EA (2022) The effect on spore viability of storing the nematode parasite Pasteuria penetrans in either dried root material or water suspension. Russ J Nematol 30(1):19–20
Tzortzakakis EA, Gowen SR (1994) Evaluation of Pasteuria penetrans alone and in combination with oxamyl, plant resistance and solarization for control of Meloidogyne spp. on vegetables grown in greenhouses in Crete. Crop Prot 13:455–462
Tzortzakakis EA, Adam MAM, Blok VC, Paraskevopoulos C, Bourtzis K (2005) Occurrence of resistant breaking populations of root-knot nematodes on tomato in Greece. Eur J Plant Pathol 113:101–105
Watson TT (2022) Sensitivity of Meloidogyne enterolobii and M. incognita to fluorinated nematicides. Pest Manag Sci 78:1398–1406. https://doi.org/10.1002/ps.6756
Wram CL, Zasada I (2020) Differential response of Meloidogyne, Pratylenchus, Globodera, and Xiphinema species to the nematicide fluazaindolizine. Phytopathology 110:2003–2009. https://doi.org/10.1094/PHYTO-05-20-0189-R
Wu HY, de Oliveira SJ, Becker JS, Becker JO (2021) Fluazaindolizine mitigates plant-parasitic nematode activity at sublethal dosages. J Pest Sci 94:573–583. https://doi.org/10.1007/s10340-020-01262-2
Funding
This project was partially funded by Corteva Agriscience. EN and AC were employed to I.O.S.V by funds of this project.
Author information
Authors and Affiliations
Contributions
EAT designed and performed the experiments and wrote the manuscript; EN contributed to part of the experimental work and analyzed the data; AC performed the experiments; TCT got the funding, designed the experiments and wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors certify that (1) do not have any actual or potential conflict of interest, (2) the study described is original, has not been published previously and is not under consideration for publication elsewhere, (3) all prevailing national and international regulations and conventions, and normal scientific ethical practices, have been respected.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tzortzakakis, E.A., Nasiou, E., Chatzaki, A. et al. Compatibility of fluazaindolizine with Pasteuria penetrans on spore attachment and infection of three Meloidogyne species. J Plant Dis Prot 130, 817–822 (2023). https://doi.org/10.1007/s41348-023-00754-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00754-4