Abstract
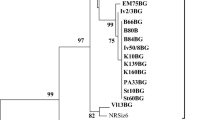
Biological and molecular properties of five Prune dwarf virus cherry isolates were studied. Double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) proved PDV infection in the respective source trees and absence of six other viruses infecting the cherry. Two of infected source trees were symptomless, and the other three trees showed deformed and elongated leaves with chlorotic lines and spots. The reaction of greenhouse-grown seedlings of 22 herbaceous test plant species was investigated. The woody indicators Prunus tomentosa and Prunus cerasifera were chip-budded. Cucumis sativus cv. Amelia, C. sativus cv. Levina, C. sativus cv. Tessa and Prunus tomentosa appeared as the most suitable test plants for bioassay of studied PDV isolates. The nucleotide sequences corresponding to the full-length coat protein (CP) of 657 bp were obtained. The identity among studied isolates in CP was 95.5–100% and 97.4–100% at nucleotide and amino acid levels, respectively. The movement protein (MP) sequences were 753 nucleotides long, translating 251 amino acids and shared 94.1–98.4% nucleotide and 96.8–99.2% amino acid identity. Constructed trees with the nucleotide sequences of both MP and CP genes of studied isolates and referent PDV cherry variants showed the formation of two phylogroups in the two genomic regions. All studied isolates separated into two clusters in group I. No correlation between the phylogenetic grouping and the biological properties, as well the geographical origin, was observed.





Similar content being viewed by others
Availability of data and material
All data that support the findings reported in this study are available from the corresponding author upon reasonable request.
References
Bachman EJ, Scott SW, Xin G et al (1994) The complete nucleotide sequences of Prune dwarf ilarvirus RNA3: implications for coat protein activation of genome replication in ilarviruses. Virology 201:127–131
Brunt AA, Crabtree K, Dallwitz MJ, Gibbs AJ, Watson L (1996) Viruses of Plants. CAB International, Wallingford
Bujarski JJ, Gallitelli D, Garcia-Arenal F, Pallas V, Palukaitis P, Reddy MK, Wand A (2019) ICTV virus taxonomy profile: Bromoviridae. J Gen Vitol 100:2006–1207. https://doi.org/10.1099/jgv.0.001282
Clark MF, Adams AN (1977) Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol 34:475–483
Cui H, Hong N, Wang G, Wang A (2013) Genomic segments RNA1 and RNA2 of Prunus necrotic ringspot virus codetermine viral pathogenicity to adapt to alternating natural Prunus hosts. MPMI 26(5):515–527. https://doi.org/10.1094/MPMI-12-12-0282-R
Damsteegt VD, Al S, Mink GI et al (1998) The versatility of Prunus tomentosa as a bioindicator of viruses. Acta Hortic 472:143–146
Flegg KL, Clark MF (1979) The detection of Apple chlorotic leaf spot virus by a modified procedure of enzyme-linked immunosorbent assay. Ann Appl Biol 91(1):61–65
Fonseca F, Neto JD, Martins V et al (2005) Genomic variability of Prune dwarf virus as affected by agricultural practice. Arch Virol 150:1607–1619
Fulton RW (1970) Prune dwarf virus. C.M.I./A.A.B. Description of Plant Viruses:19 (Unavailable online)
Garcia JA, Pallas V (2015) Viral factors involved in plant pathogenesis. Curr Opin Virol 11:21–30
Halk EI, Fulton RW (1978) Stabilization and particle morphology of Prune dwarf virus. Virology 91:434–443
Higgins D, Thompson J, Gibson T et al (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Kalinowska E, Mroczkowska K, Paduch-Cichal E, Chrorska M (2014) Genetic variability among coat protein of Prune dwarf virus variants from different countries and different Prunus species. Eur J Plant Pathol 140:863–868
Kamenova I, Borisova A, Popov A (2019) Incidence and genetic diversity of Prune dwarf virus in sweet and sour cherry in Bulgaria. Biotech Biotech Eq 33(1):980–987. https://doi.org/10.1080/13102818.2019.1637278
Kamenova I, Borisova A, Popov A (2020) Occurrence of Ilarviruses in sweet and sour cherry in Bulgaria. Bulg J Agric Sci 26(3):590–597
Koziel E, Otulak K, Garbaczewska G (2015) Phylogenetic analysis of PDV movement protein compared to Bromoviridae members as justification of possible intracellular movement. Acta Biol Crac Series Bot 57(2):106–114
Koziel E, Bujarski JJ, Otulak K (2017) Molecular biology of Prune dwarf virus - A lesser known member of the Bromoviridae but a vital component in the dynamic virus-host cell interaction network. Int J Mol Sci 18:2733
Kunze L, Clark MF, Flegg CL (1984) Comparison of methods for characterizing and distinguishing isolates of Prune dwarf virus. Phytopath Z 110:251–260
Massart S, Brostaux Y, Barbarossa L et al (2008) Inter-laboratory evaluation of a duplex RT-PCR method using crude extracts for the simultaneous detection of Prune dwarf virus and Prunus necrotic ringspot virus. Eur J Plant Pathol 122:539–547
Mink GI (1993) Pollen and seed-transmitted viruses and viroids. Annu Rev Phytopathol 31:375–402
Nemeth M (1986) Virus, mycoplasma and rikettsia diseases of fruit trees. Academia Kiado, London
Ȫztürk Y, Cevik B (2015) Genetic diversity in the coat protein genes of Prune dwarf virus isolates from sweet cherry growing in Turkey. Plant Pathol J 31(1):41–49
Paduch-Cichal E (2000) Characterization of PNRSV and PDV. Associate Professor Thesis, Warsaw University of Live Sciences, Warsaw, Poland
Paduch-Cichal E, Sala-Rejczak K, Mroczkiowska K et al (2011) Serological characterization of Prune dwarf virus isolates. J Pant Protect Res 51(4):389–392
Pallas V, Aparicio F, Herranz MC et al (2013) Molecular biology of Ilarviruses. Adv Virus Res 87:139–181
Predajňa L, Sihelská N, Benediková D et al (2017) Molecular characterization of Prune dwarf virus cherry isolates from Slovakia events shows their substantial variability and reveals recombination. Eur J Plant Pathol 147:877–885
Šutic DD, Ford RE, Tosic MT (1999) Virus diseases of fruit trees, In: Handbook of plant virus diseases, CRC Press, Boca Raton, FL, USA
Tamura K, Stecher G, Peterson D et al (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Ulubaş-Serçe Ç, Ertunç F, Oeztuerk A (2009) Identification and genomic variability of Prune dwarf virus variants infecting stone fruit trees in turkey. J Phytopathol 157:298–305
Vaskova D, Pertzik K, Spak J (2000) Molecular variability of the capsid protein of the Prune dwarf virus. Eur J Plant Pathol 106:573–580
Waterworth HE, Fulton RW (1964) Variation among isolates of necrotic ringspot and prune dwarf viruses isolated from sour cherry. Phytopathology 54:1155–1160
Funding
This research was supported by National Science Fund, Ministry of Education and Science, Bulgaria, grant number DH16/7 dated 11.12.2017.
Author information
Authors and Affiliations
Contributions
IK performed molecular analysis and drafted the manuscript. AB performed biological assays.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamenova, I.L., Borisova, A.Z. Biological and molecular properties of Prune dwarf virus cherry isolates from Bulgaria. J Plant Dis Prot 129, 301–311 (2022). https://doi.org/10.1007/s41348-021-00551-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-021-00551-x