Abstract
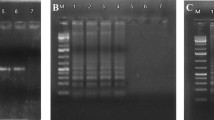
Recombinase Polymerase Amplification (RPA) assay was developed for specific detection of Tomato leaf curl New Delhi virus-potato (ToLCNDV [potato]), causing potato apical leaf curl disease. The RPA assay was optimized with respect to its isothermal temperature, post-amplification treatment and its components of the reaction mix. The assay showed a clear and sharp expected amplicon at 40 °C for 30 min in the thermal cycle followed by post-amplification denaturation, i.e., heat treatment at 65 °C for 10 min. The target specificity of the developed assay was determined by sequencing the amplified product. Pairwise sequence alignment showed 73 numbers of significant hits with ToLCNDV [potato] isolates within the taxonomy of begomovirus with query coverage of 100% with 99% identity indicating that the assay is specific to ToLCNDV [potato]. The comparative sensitivity of the assay was ten times more sensitive than PCR. It was successfully applied for the detection of ToLCNDV [potato] from potato leaf samples, tissue culture raised plants and also from tuber and sprouts. The assay was proved to be rapid, sensitive, highly specific and suitable in diagnostic laboratories for virus indexing and selection of virus-free planting material. To the best of our knowledge, this is the first report of the development and application of RPA assay for the detection of ToLCNDV [potato].








Similar content being viewed by others
References
Babu B, Washburn BK, Miller SH, Poduch K, Sarigul T, Knoxa GW, Ochoa-Corona FM, Paret ML (2017) A rapid assay for detection of Rose rosette virus using reverse transcription-recombinase polymerase amplification using multiple gene targets. J Virol Meth 240:78–84
Chandel RS, Banyal DK, Singh BP, Malik K, Lakra BS (2010) Integrated management of whitefly Bemisiatabaci (Gennadius) and Potato apical leaf curl virus in India. Pot Res 53:129–139
Chandu D, Paul S, Parker M, Dudin Y, King-Sitzes J, Perez T, Mittanck DW, Shah M, Glenn KC, Piepenburg O (2016) Development of a rapid point-of-use DNA test for the screening of Genuity® roundup ready 2 Yield® soybean in seed samples. Biomed Res Inter. https://doi.org/10.1155/2016/3145921
Crannell ZA, Rohrman B, Richards-Kortum R (2014) Equipment-free incubation of recombinase polymerase amplification reactions using body heat. PLoS One. https://doi.org/10.1371/journal.pone.0112146
Daher RK, Stewart G, Boissinot M, Boudreau DK, Bergeron MG (2016) Recombinase polymerase amplification for diagnostic applications. ClinChem 62:947–958
Fuller SL, Savory EA, Weisberg AJ, Buser JZ, Gordon MI, Putnam ML, Chang JH (2017) Isothermal amplification and lateral-flow assay for detecting crown-gall-causing Agrobacteriumspp. Phytopath 107:1062–1068
Garg ID, Khurana SMP, Kumar S, Lakra BS (2001) Association of a geminivirus with potato apical leaf curl in India and its immunized electron microscopic detection. J Ind Pot Asso 28:227–232
Glais L, Jacquot E (2015) Detection and characterization of viral species/ subspecies using isothermal recombinase polymerase amplification (RPA) assays. Methods MolBiol 130(2):207–225
Hong-mei W, Gui-min Z, Pei-li H, Li Y, Cheng-qiang H, Hong-bin H (2018) Rapid detection of foot-and-mouth disease virus using reverse transcription recombinase polymerase amplification combined with lateral flow dipstick. J Virol Meth 261:46–50
Jeevalatha A, Kaundal P, Venkatasalam EP, Chakrabarti SK, Singh BP (2013) Uniplex and duplex PCR detection of geminivirus associated with potato apical leaf curl disease in India. J Virol Meth 193:62–67
Jeevalatha A, Singh BP, Kaundal P, Kumar R, Raigond B (2014) RCA-PCR: a robust technique for the detection of Tomato leaf curl New Delhi virus-[potato] at ultra-low virus titre. Potato J 41:76–80
Jeevalatha A, Kaundal P, Kumar A, Guleria A, Sundaresha S, Pant RP, Sridhar J, Venkateswarlu V, Singh BP (2016) SYBR green-based duplex RT-qPCR detection of a begomovirus, Tomato leaf curl New Delhi virus-[potato] along with Potato virus X and Potato leaf roll virus in potato. Potato J 43:125–137
Jeevalatha A, Chakrabarti SK, Sharma S, Sagar V, Malik K, Raigond B, Singh BP (2017) Diversity analysis of tomato leaf curl New Delhi virus-[potato], causing apical leaf curl disease of potato in India. Phytoparasitica 45:33–43
Jia L, Joanne M, Felix VS (2019) Review: a comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 144:31–67
Jiao Y, Jiang J, An M, Xia Z, Wu Y (2019) Recombinase polymerase amplification assay for rapid detection of Maize chloroticmottel virus in maize. Arc Virol 164(10):2581–2584
Kersting S, Rausch V, von Bier FF, Nickisch-Rosenegk M (2014) Rapid detection of Plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis. Malar J 13:99
Lakra BS (2002) Leaf curl: a threat to potato crop in Haryana. J Myco Pl Pathol 32:367
Lakra BS (2003) Potato apical leaf curl begomovirus-symptoms, appraisal of a scale and losses in potato crop. J Ind Pot Asso 30:119–120
Lillis L, Lehman D, Singhal MC, Cantera J, Singleton J, Labarre P, Toyama A, Piepenburg O, Parker M, Wood R, Overbaugh J, Boyle DS (2014) Non instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA. PLoS One 9(9):e108189
Liu Q, Lim BKL, Lim YS, Tang WY, Gu Z, Chung J, Park MK, Barkham T (2018) Label-free, real-time and multiplex detection of Mycobacterium tuberculosis based on silicon photonic microring sensors and asymmetric isothermal amplification technique (SPMS-AIA). Sens Actuators B 255:1595–1603
Lobato IM, O’Sullivan CK (2018) Recombinase polymerase amplification: basics, applications and recent advances. Trend Anal Chem 98:19–35
Londono MA, Harmon CL, Polston JE (2016) Evaluation of recombinase polymerase amplification for detection of begomoviruses by plant diagnostic clinics. Virol J 22:13–48
Martorell S, Palanca S, Maquieira A, Tortajada-Genaro, (2018) Blocked recombinase polymerase amplification for mutation analysis of PIK3CA gene. Anal Biochem 544:49–56
Mayboroda O, Benito AG, Del Rio JS, Svobodova M, Julich S, Tomaso H, O’Sullivan CK, Katakis I (2015) Isothermal solid-phase amplification system for detection of Yersinia pestis. Anal BioanalChem 408:671–676
Mekuria TA, Zhang S, Eastwella KC (2014) Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification Tefera. J Virol Meth 205:24–30
Piepenburg O, Williams CH, Stemple DL, Armes NA (2006) DNA detection using recombinase proteins. PLoSBiol 4:1115–1121
Qinglin M, Houming L, Feidi Y, Guangxin X, Wanshui S, Wanli X (2017) Rapid and visual detection of Mycobacterium tuberculosis complex using recombinase polymerase amplification combined with lateral flow strips. MolCellu Probes 36:43–49
Reetika K, Nishant S, Shailender K, Saritha RK, Sharma SK, Jain RK, Baranwal VK (2017) Development of a recombinase polymerase amplification assay for the diagnosis of Banana bunchy top virus in different banana cultivars. Arc Virol 162(9):2791–2796
Saha A, Saha B, Saha D (2014) Molecular detection and partial characterization of a begomovirus causing leaf curl disease of potato in sub-Himalayan West Bengal, India. J EnvirBiol 35:601–606
Silva G, Oyekanmi J, Nkere CK, Bömer M, Kumar PL, Seal SE (2018) Rapid detection of potyviruses from crude plant extracts. Ana Biochem 546:17–22
Sohrab SS, Karim S, Varma A, Abuzenadah AM, Chaudhary AG, Mandal B (2013) Role of sponge gourd in apical leaf curl disease of potato in Northern India. Phytopara 41:403–410
Usharani KS, Surendranath B, Khurana SMP, Garg ID, Malathi VG (2004) Potato leaf curl – a new disease of potato in northern India caused by a strain of Tomato leaf curl New Delhi virus. Pl Pathol 53:235
Varma A, Malathi VG (2003) Emerging geminivirus problems: a serious threat to crop production. Ann App Biol 142:145–164
Venkatasalam EP, Singh S, Sivalingam PN, Malathi VG, Garg ID (2011) Polymerase chain reaction and nucleic acid spot hybridization detection of begomovirus (es) associated with apical leaf curl disease of potato. Arch Phytopathol Pl Protec 44:987–992
Wang R, Zhang F, Wang L, Qian W, Qian C, Wu J, Ying Y (2017) Instant, visual and instrument-free method for on-site screening of GTS 40-3-2 soybean based on body-heat triggered recombinase polymerase amplification. Anal Chem 89:4413–4418
Xia X, Yu Y, Weidmann M, Pan Y, Yan S, Wany Y (2014) Rapid detection of shrimp white spot syndrome virus by real-time, isothermal recombinase polymerase amplification assay. Plos One 9(8):e104667
Yamanaka ES, Tortajada-Genaro LA, Maquieira A (2017) Low-cost genotyping method based on allele-specific recombinase polymerase amplification and colorimetric microarray detection. MicrochimActa 184:1453–1462
Zhang S, Ravelonandrom M, Russell P, McOwen N, Briard P, Bohannon S, Vrient A (2014) Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP® using reverse transcription-recombinase polymerase amplification. J Virol Meth 207:114–120
Acknowledgements
The authors are thankful to Indian Council of Agricultural Research (ICAR), New Delhi, India for funding a project on “Development and application of Diagnostics to virus, viroid and phytoplasma infecting Potato” (Project Number/ Code. 1009850) through a “Consortium Research Platform (CRP) on Vaccines and Diagnostics.”
Funding
This study was funded by Indian Council of Agricultural Research (ICAR), New Delhi, India through Consortium Research Platform (CRP) on Vaccines and Diagnostics (Project No/ Grant No. 1009850).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Human and animals rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raigond, B., Pathania, S., Verma, A. et al. Recombinase Polymerase Amplification assay for rapid detection of a geminivirus associated with potato apical leaf curl disease. J Plant Dis Prot 128, 1061–1071 (2021). https://doi.org/10.1007/s41348-021-00466-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-021-00466-7