Abstract
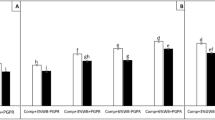
The aim of this work was to assess the potential suppression of three on-farm green composts for controlling seven soil-borne pathogens in container media under greenhouse condition. Suppression of Pythium irregulare and Rhizoctonia solani damping-off of cucumber and bean, Phytophthora cinnamomi and Sclerotinia minor root rot of azalea and lettuce, and Fusarium oxysporum wilt of melon, tomato, and basil was studied on artificially inoculated seedlings. Three feedstocks of bioenergy wastes and agricultural residues were selected, on-farm composted, characterized, and tested for their suppressive properties by in vitro and in vivo experiments in comparison with one commercial compost from municipal solid biowaste. The composts showed differences in the suppressive properties when mixed with sterile peat at dosage of 35%. All green composts meanly suppressed P. irregulare damping-off of cucumber of 80%, R. solani damping-off of bean of 75%, P. cinnamomi root rot of azalea of 65%, and Fusarium wilt of up to 25%. The reference compost suppressed F. oxysporum wilt of melon, tomato, and basil from 60 to 70% and Pythium, Rhizoctonia, and Phytophthora diseases up to 30%. All composts suppressed S. minor root rot of lettuce of 35%. Suppression of Pythium damping-off and Phytophthora root rot was related to the sum of the bioactivities of the fungi and bacteria of compost. Suppression of Rhizoctonia damping-off and F. oxysporum wilt was associated with the specific bioactivity of a restricted number of fungi (Trichoderma, Aspergillus) and bacteria (Pseudomonas, actinomycetes) species. Suppression of Sclerotinia root rot was not related to any one variable of composts.




Similar content being viewed by others
References
Alfano G, Lustrato G, Lima G, Vitullo D, Delfine S, Tognetti R, Ranalli G (2009) Physical-chemical, microbiological, agronomical and phytopathological aspects in the recycling of olive waste composted residues. Dyn Soil Dyn Plant 3(2):64–72
Avilés M, Borrero C, Trillas MI (2011) Review on compost as an inducer of disease suppression in plants grown in soilless culture. Dyn Soil Dyn Plant 5(2):1–11
Boehm MJ, Hoitink HAJ (1992) Sustenance of microbial activity in potting mixes and its impact on severity of Pythium root rot of poinsettia. Phytopathology 82:259–264
Bonanomi G, Antignani V, Pane C, Scala F (2007) Suppression of soil-borne fungal diseases with organic amendments. J Plant Pathol 89:311–324
Bonanomi G, Antignani V, Capodilupo M, Scala F (2010) Identifying the characteristics of organic soil amendments that suppress soil-borne plant diseases. Soil Biol Biochem 42:136–144
Bonilla N, Gutierrez-Barranquero JA, De Vicente A, Cazorla FM (2012) Enhancing soil quality and plant health through suppressive organic amendments. Diversity 4:475–491
Borrero C, Trillas MI, Ordovás J, Tello JC, Avilés M (2004) Predictive factors for the suppression of Fusarium wilt of tomato in plant growth media. Phytopathology 94:1094–1101
Bugbee WM (1990) Purification and characteristics of pectin lyase from Rhizoctonia solani. Physiol Mol Plant Pathol 36:15–25
Chen W, Hoitink HAJ, Madden LV (1988a) Microbial activity and biomass in container media for predicting suppressiveness to damping-off caused by Pythium ultimum. Phytopathology 78:1447–1450
Chen WD, Hoitink HAJ, Schmitthenner AF, Tuovinen OH (1988b) The role of microbial activity in suppression of damping-off caused by Pythium ultimum. Phytopathology 78:314–322
Chet I, Baker R (1980) Induction of suppressiveness to Rhizoctonia solani in soil. Phytopathology 70:994–998
Chilosi G, Aleandri MP, Bruni N, Tomassini A, Torresi V, Muganu M, Paolocci M, Vettraino A, Vannini A (2017) Assessment of suitability and suppressiveness of on-farm green compost as a substitute of peat in the production of lavender plants. Biocontrol Sci Tech. doi:10.1080/09583157.2017.1320353
Craft CM, Nelson EB (1996) Microbial properties of composts that suppress damping-off and root rot of creeping bent grass caused by Pythium graminicola. Appl Environ Microbiol 62:1550–1557
De Corato U, Viola E, Arcieri G, Valerio V, Zimbardi F (2016) Use of composted agro-energy co-products and agricultural residues against soil-borne pathogens in horticultural soil-less systems. Sci Hortic 210:166–179
de la Cruz JMR, Lora JM, Hidalgo-Galiego A, Dominguez F, Pintor-Toro JA, Llobell A, Benitez T (1993) Carbon source control on β-glucanases, chitobiase and chitinase from Trichoderma harzianum. Microbiology 159:316–322
Diab HG, Hu S, Benson DM (2003) Suppression of Rhizoctonia solani on impatiens by enhanced microbial activity in swine waste-amended potting mixes. Phytopathology 93:1115–1123
Elad Y, Chet I (1983) Improved selective media for isolation of Trichoderma spp. or Fusarium spp. Phytoparasitica 11:55–58
El-Masry MH, Khalil AI, Hassouna MS, Ibrahim HAH (2002) In situ and in vitro suppressive effect of agricultural composts and their water extracts on some phytopathogenic fungi. World J Microbiol Biotechnol 18:551–558
Hadar Y, Papadopoulou KK (2012) Suppressive composts: microbial ecology links between abiotic environments and healthy plants. Annu Rev Phytopathol 50:133–153
Hardy GE, Sivasithamparam K (1991) Suppression of Phytophthora root rot by a composted eucalyptus bark mix. Aust J Bot 39:153–159
Inbar Y, Boehm MJ, Hoitink HAJ (1991) Hydrolysis of fluorescein diacetate in sphagnum peat container media for predicting suppressiveness to damping-off caused by Pythium ultimum. Soil Biol Biochem 23:479–483
Inbar Y, Chen Y, Hoitink HAJ (1993) Properties for establishing standards for utilization of composts in container media. In: Hoitink HAJ, Keener HM (eds) Science and engineering of composting. Renaissance Publications, Worthington, pp 601–621
Kinkel LL, Schlatter DC, Bakker MG, Arenz BE (2012) Streptomyces competition and co-evolution in relation to plant disease suppression. Resistance Microbiol 163:490–499
Kuter GA, Nelson EB, Hoitink HAJ, Madden LV (1983) Fungal populations in container media amended with composted hardwood bark suppressive and conductive to Rhizoctonia damping-off. Phytopathology 73:1450–1456
Kuter GA, Hoitink HAJ, Chen W (1988) Effects of municipal sludge compost curing time on suppression of Pythium and Rhizoctonia diseases of ornamental plants. Plant Dis 72:751–756
Kwok OCH, Fahy PC, Hoitink HAJ, Kuter GA (1987) Interactions between bacteria and Trichoderma hamatum in suppression of Rhizoctonia damping-off in bark compost media. Phytopathology 77:1206–1212
Larkin RP (2015) Soil health paradigms and implications for disease management. Ann Rev Phytopathol 53:199–221
Malandraki I, Tjamos SE, Pantelides IS, Paplomatas EJ (2008) Thermal inactivation of compost suppressiveness implicates possible biological factors in disease management. Biol Control 44:180–187
Manici LM, Caputo F, Babini V (2004) Effect of green manure on Pythium spp. population and microbial communities in intensive cropping systems. Plant Soil 263:133–142
Nelson EB, Hoitink HAJ (1982) Factors affecting suppression of Rhizoctonia solani in container media. Phytopathology 72:275–279
Nelson EB, Kuter GA, Hoitink HAJ (1983) Effects of fungal antagonists and compost age on suppression of Rhizoctonia damping-off in container media amended with composted hardwood bark. Phytopathology 73:1457–1462
Nguyen NV, Kim YY, Oh KT, Jung WJ, Park RD (2008) Antifungal activity of chitinases from Trichoderma aureoviride DY-59 and Rhizopus microsporus VS-9. Curr Microbiol 56:28–32
Noble R (2011) Risks and benefits of soil amendment with composts in relation to plant pathogens. Aust Plant Pathol 40:157–167
Noble R, Coventry E (2005) Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci Technol 15:3–20
Ocamb CM, Kommedahl T (1994) Growth of rhizosphere competent and in-competent Fusarium species from corn on carbon substrates. Phytopathology 84:508–514
Pane C, Spaccini R, Piccolo A, Scala F, Bonanomi G (2011) Compost amendments enhance peat suppressiveness to Pythium ultimum, Rhizoctonia solani and Sclerotinia minor. Biol Control 56:115–124
Pane C, Piccolo A, Spaccini R, Celano G, Villecco D, Zaccardelli M (2013) Agricultural waste-based composts exhibiting suppressivity to diseases caused by the phytopathogenic soil-borne fungi Rhizoctonia solani and Sclerotinia minor. Appl Soil Ecol 65:43–51
Pane C, Celano G, Piccolo A, Villecco D, Spaccini R, Palese AM, Zaccardelli M (2015) Effects of on-farm composted tomato residues on soil biological activity and yields in a tomato cropping system. Chem Biol Technol Agric. doi:10.1186/s40538-014-0026-9
Russo G, Verdiani G, Anifantis AS (2016) Re-use of agricultural biomass for nurseries using proximity composting. Contemp Eng Sci 9:1151–1182
Saadi I, Laor Y, Medina S, Krassnowsky A, Raviv M (2010) Compost suppressiveness against Fusarium oxysporum was not reduced alter one-year storage under various moisture and temperature conditions. Soil Biol Biochem 42:626–634
Scheuerell SJ, Sullivan DM, Mahaffee WF (2005) Suppression of seedling damping-off caused by Pythium ultimum, P. irregulare, and Rhizoctonia solani in container media amended with a diverse range of Pacific Northwest compost sources. Phytopathology 95:306–315
Scotti R, Pane C, Spaccini R, Palese AM, Piccolo A, Celano G, Zaccardelli M (2016) On-farm compost: a useful tool to improve soil quality under intensive farming systems. Appl Soil Ecol 107:13–23
Suàrez-Estrella F, Vargas-Garcìa C, Lopez MJ, Capel C, Moreno J (2007) Antagonistic activity of bacteria and fungi from horticultural compost against Fusarium oxysporum f. sp. melonis. Crop Protect 26:46–53
Termorshuizen AJ, Jeger MJ (2008) Strategies of soilborne plant pathogenic fungi in relation to disease suppression. Fungal Ecol 1:108–114
Trillas MI, Hoitink HAJ, Madden LV (1986) Nature of suppression of Fusarium wilt of radish in a container medium amended with composted hardwood bark. Plant Dis 70:1023–1027
Trillas MI, Casanova E, Cotxarrera L, Ordovás J, Borrero C, Avilés M (2006) Composts from agricultural waste and the Trichoderma asperellum strain T-34 suppress Rhizoctonia solani in cucumber seedlings. Biol Control 39:32–38
Tuitert G, Szczech M, Bollen GJ (1998) Suppression of Rhizoctonia solani in potting mixtures amended with compost made from organic household waste. Phytopathology 88:764–773
Acknowledgements
The authors are thankful to Luigi Patruno, Ph.D., and Dr. Nicola Avella of BIO-PLANTA Research Consortium for their useful contribution to the success of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflict of interest
All authors declare that no conflict of interest exists in this research work.
Rights and permissions
About this article
Cite this article
De Corato, U., Salimbeni, R. & De Pretis, A. Suppression of soil-borne pathogens in container media amended with on-farm composted agro-bioenergy wastes and residues under glasshouse condition. J Plant Dis Prot 125, 213–226 (2018). https://doi.org/10.1007/s41348-017-0133-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-017-0133-5