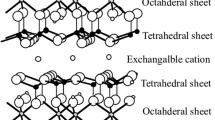
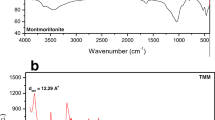
Abstract
There is an increasing demand for practical adsorbents with low cost, high availability, and sustainability, to adsorb and remove radioactive technetium materials. It is imperative to develop high-performance substances that are widely available in nature. In the present study, we used three different natural claystone samples collected from three different areas in the Eastern Desert, Egypt. Cetyltrimethylammonium bromide (CTAB) was mixed with the raw clay samples to prepare modified organoclay, which was used in the removal of technetium. Raw and modified samples were investigated by X-ray diffraction (XRD) analysis, scanning electron microscopy (SEM), and Fourier-transform infrared (FT-IR) spectroscopy. The prepared organoclay samples were then used to remove radioactive technetium materials, applied for healthcare and provided by the Upper Egypt Cancer Institute. In the adsorption processes, three parameters were studied (catalyst weight, contact time, and initial concentration of technetium). Also, kinetic and isothermal models were studied to describe how the adsorption process occurs. The obtained data revealed a maximum removal of 59.4%, 83.6%, and 87.2% for catalyst weight of 0.5 g, 0.4 g, and 0.4 g when using O-Uh2, O-H25, and O-Sy4, respectively. Moreover, the adsorption process followed the pseudo-second-order and intraparticle diffusion kinetic models, while in isothermal studies the adsorption process followed the Freundlich model but not the Langmuir model. This indicates a monolayer with chemical sharing and/or ion exchange formed during the adsorption process.












Similar content being viewed by others
Data availability
The data that used in this study is available on reasonable request.
References
Ahmed AS, Hassan WA, Ahmed EA et al (2023) Ultra-fast adsorption of radioactive technetium (99mTc) by using mining waste Clay samples, Abu-Tartur, Egypt. Sci Rep. https://doi.org/10.1038/s41598-023-42757-z
Ahmed Said AEA, Goda MN (2021) Superior competitive adsorption capacity of natural bentonite in the efficient removal of basic dyes from aqueous solutions. ChemistrySelect 6:2790–2803. https://doi.org/10.1002/slct.202100575
Arai T, Wei Y, Kumagai M, Horiguchi K (2006) Separation of rare earths in nitric acid medium by a novel silica-based pyridinium anion exchange resin. J Alloys Compd 408:1008–1012
Asuquo ED, Martin AD (2016) Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: characterisation, kinetic and isotherm studies. J Environ Chem Eng 4:4207–4228. https://doi.org/10.1016/j.jece.2016.09.024
Blanco SPDM, Scheufele FB, Módenes AN et al (2017) Kinetic, equilibrium and thermodynamic phenomenological modeling of reactive dye adsorption onto polymeric adsorbent. Chem Eng J 307:466–475
Bonnesen PV, Brown GM, Alexandratos SD et al (2000) Development of bifunctional anion-exchange resins with improved selectivity and sorptive kinetics for pertechnetate: batch-equilibrium experiments. Environ Sci Technol 34:3761–3766
Bors J, Dultz S, Riebe B (2000) Organophilic bentonites as adsorbents for radionuclides: I. Adsorption of ionic fission products. Appl Clay Sci 16:1–13
Del Cul GD, Bostick WD, Trotter DR, Osborne PE (1993) Technetium-99 removal from process solutions and contaminated groundwater. Sep Sci Technol 28:551–564
Dickson JO, Harsh JB, Flury M et al (2014) Competitive incorporation of perrhenate and nitrate into sodalite. Environ Sci Technol 48:12851–12857
Eiroa-Lledo C, Lecrivain L, Parker TG et al (2020) Comparison of ReO4− and TcO4− in solvent extraction systems. Radiochim Acta 108:443–449
Freundlich H (1906) Concerning adsorption in solutions. Z Phys Chem 57:385–470
Gu B, Dowlen KE, Liang L, Clausen JL (1996) Efficient separation and recovery of technetium-99 from contaminated groundwater. Sep Technol 6:123–132
Gu B, Brown GM, Bonnesen PV et al (2000) Development of novel bifunctional anion-exchange resins with improved selectivity for pertechnetate sorption from contaminated groundwater. Environ Sci Technol 34:1075–1080
Hercigonja RV, Vranješ-Djurić SD, Mirković MD et al (2018) Technetium removal from the aqueous solution using zeolites A and Y containing transition metal ions Co2+ and Zn2+. J Radioanal Nucl Chem 317:215–225
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Icenhower JP, Qafoku NP, Zachara JM, Martin WJ (2010) The biogeochemistry of technetium: a review of the behavior of an artificial element in the natural environment. Am J Sci 310:721–752
Ito K, Kanno T (1988) Sorption behavior of carrier-free technetium-95m on minerals, rocks and backfill materials under both oxidizing and reducing conditions. J Nucl Sci Technol 25:534–539
Jurisson S, Gawenis J, Landa ER (2004) Sorption of 99mTc radiopharmaceutical compounds by soils. Health Phys 87:423–428
Kaplan DI, Jeffrey R (1998) Pertechnetate exclusion from sediments. Radiochim Acta 81:117–124
Kumar KV, Sivanesan S, Ramamurthi V (2005) Adsorption of malachite green onto Pithophora sp., a fresh water algae: equilibrium and kinetic modelling. Process Biochem 40:2865–2872
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li J, Dai X, Zhu L et al (2018) 99TcO4—remediation by a cationic polymeric network. Nat Commun 9:1–11
Lieser KH, Bauscher C (1988) Technetium in the hydrosphere and in the geosphere. Pt. 2. Radiochim Acta 44:125–128
Liu Y, Terry J, Jurisson SS (2007) Pertechnetate immobilization in aqueous media with hydrogen sulfide under anaerobic and aerobic environments. Radiochim Acta 95:717–725
Liu T, Li Y, Du Q et al (2012) Adsorption of methylene blue from aqueous solution by graphene. Colloids Surfaces B Biointerfaces 90:197–203
Ma Y, Zhu J, He H et al (2010) Infrared investigation of organo-montmorillonites prepared from different surfactants. Spectrochim Acta Part A Mol Biomol Spectrosc 76:122–129
Madejová J (2003) FTIR techniques in clay mineral studies. Vib Spectrosc 31:1–10
Miniakhmetov IA, Semenov SA, Musatova VY, Reznik AM (2013) Solvent extraction of rhenium with N-(2-hydroxy-5-nonylbenzyl)-β-hydroxyethylmethylamine. Russ J Inorg Chem 58:1380–1382
Mishra AK, Allauddin S, Narayan R et al (2012) Characterization of surface-modified montmorillonite nanocomposites. Ceram Int 38:929–934
Momoshima N, Sayad M, Takashima Y (1995) Determination of 99 Tc in coastal seawater collected in Fukuoka, Japan. J Radioanal Nucl Chem 197:245–251
Momoshima N, Sayad M, Yamada M et al (2005) Global fallout levels of 99 Tc and activity ratio of 99 Tc/137 Cs in the Pacific Ocean. J Radioanal Nucl Chem 266:455–460
Peretroukhine V, Sergeant C, Devès G et al (2006) Technetium sorption by stibnite from natural water. Radiochim Acta 94:665–669
Pierce EM, Lilova K, Missimer DM et al (2017) Structure and thermochemistry of perrhenate sodalite and mixed guest perrhenate/pertechnetate sodalite. Environ Sci Technol 51:997–1006
Sheng D, Zhu L, Xu C et al (2017) Efficient and selective uptake of TcO4–by a cationic metal–organic framework material with open Ag+ sites. Environ Sci Technol 51:3471–3479
Shi K, Hou X, Roos P, Wu W (2012) Determination of technetium-99 in environmental samples: a review. Anal Chim Acta 709:1–20
Shi K, Ye Y, Guo N et al (2014) Evaluation of Se (IV) removal from aqueous solution by GMZ Na-bentonite: batch experiment and modeling studies. J Radioanal Nucl Chem 299:583–589
Shirzad-Siboni M, Khataee A, Hassani A, Karaca S (2015) Preparation, characterization and application of a CTAB-modified nanoclay for the adsorption of an herbicide from aqueous solutions: kinetic and equilibrium studies. C R Chim 18:204–214
Sugashini S, Sheriffa Begum KM (2013) Column adsorption studies for the removal of Cr (VI) ions by ethylamine modified chitosan carbonized rice husk composite beads with modelling and optimization. J Chem 2013:1–11
Swenson AH, Stadie NP (2019) Stadie_Langmuir_2019_FINAL.pdf. 16:5409–5426. https://doi.org/10.1021/acs.langmuir.9b00154.Made
Till JE (1986) Source terms for technetium-99 from nuclear fuel cycle facilities. Technetium in the environment. Springer, pp 1–20
Um W, Chang H-S, Icenhower JP et al (2011) Immobilization of 99-technetium (VII) by Fe (II)-goethite and limited reoxidation. Environ Sci Technol 45:4904–4913
Vandergraaf TT, Ticknor KV, George IM (1984) Reactions between technetium in solution and iron-containing minerals under oxic and anoxic conditions. ACS Publications
Volkert WA, Jurisson S (1996) Technetium-99m chelates as radiopharmaceuticals. Technetium rhenium their chem its appl. Springer, pp 123–148
Wang Y, Gao H (2006) Compositional and structural control on anion sorption capability of layered double hydroxides (LDHs). J Colloid Interface Sci 301:19–26
Wang P-Y, Zu J-H, Wei Y-Z (2017) Synthesis and characterization of porous 4VP-based adsorbent for Re adsorption as analogue to 99Tc. Nucl Sci Tech 28:1–7
Wang X, Hu X, Song L et al (2020) Efficient separation of perrhenate as analogue to pertechnetate in nitric acid solution with a DOTA-tetraamide ligand: Solvent extraction, complexation and structure study. J Mol Struct 1216:128330
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59
Xia M, Jiang Y, Li F et al (2009) Preparation and characterization of bimodal mesoporous montmorillonite by using single template. Colloids Surfaces A Physicochem Eng Asp 338:1–6
Xiao C, Silver MA, Wang S (2017) Metal–organic frameworks for radionuclide sequestration from aqueous solution: a brief overview and outlook. Dalt Trans 46:16381–16386
Yang J, Shi K, Gao X et al (2020) Hexadecylpyridinium (HDPy) modified bentonite for efficient and selective removal of 99Tc from wastewater. Chem Eng J 382:122894
Yang J, Shi K, Wu F et al (2022) Technetium-99 decontamination from radioactive wastewater by modified bentonite: batch, column experiment and mechanism investigation. Chem Eng J 428:131333. https://doi.org/10.1016/j.cej.2021.131333
Zengnian S, Minghua Y (2010) Adsorption of rhenium (VII) with anion exchange resin D318. Chin J Chem Eng 18:372–376
Zhao D, Chen S, Yang S et al (2011) Investigation of the sorption behavior of Cd (II) on GMZ bentonite as affected by solution chemistry. Chem Eng J 166:1010–1016
Zhu J, He H, Zhu L et al (2005) Characterization of organic phases in the interlayer of montmorillonite using FTIR and 13C NMR. J Colloid Interface Sci 286:239–244
Zhu L, Sheng D, Xu C et al (2017) Identifying the recognition site for selective trapping of 99TcO4–in a hydrolytically stable and radiation resistant cationic metal–organic framework. J Am Chem Soc 139:14873–14876
Funding
There is no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Responsible Editor: Mongi Seffen.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, A.S., Dardir, F.M., Ahmed, E.A. et al. Removal of technetium (99mTc) using modified claystone (organoclay). Euro-Mediterr J Environ Integr 9, 95–104 (2024). https://doi.org/10.1007/s41207-023-00452-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41207-023-00452-5