Abstract
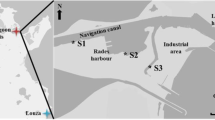
Immunological alterations may be used as ecotoxicological biomarkers to detect and monitor biological effects of chemical contaminants in polluted environments. The main goal of this study was to assess the immunotoxic effects of hydrocarbon contamination in the soft tissues of carpet shell clams (Ruditapes decussatus) sampled from the South Lagoon of Tunis (Tunisia). Sediments and clams were collected from four locations, three of which were located within the polluted South Lagoon of Tunis (S1, S2 and S3) whereas the other was a clean site on the Mediterranean coast. Total hydrocarbons were extracted from the clam tissues and sediments and fractionated into aromatic and nonaromatic hydrocarbons. The status of the clam immune system was assessed by measuring the total haemocyte count (THC) and the phenoloxidase, lysozyme and esterase activities in haemolymph samples from the clams. Hydrocarbon analysis revealed that pollution levels in the South Lagoon of Tunis were high and spatially variable. Phenoloxidase activity was inhibited in clams sampled at sites S1, S2 and S3 compared to clams at the control site, while lysozyme and esterase activities and THCs were higher in the clams at the polluted sites than in those at the control site. Moreover, the biomarker responses indicated that S3 was the most polluted site in the South Lagoon of Tunis. The results of the present study clearly show that suitable biomarkers can be useful tools for biomonitoring in the study area.






Similar content being viewed by others
References
Abdel-Shafy HI, Mansour MS (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123
Abrajano T Jr, Yan B, O'Malley V (2003) High molecular weight petrogenic and pyrogenic hydrocarbons in aquatic environments. Treatise Geochem 9:612
Ahmad I, Coelho JP, Mohmood I, Pacheco M, Santos MA, Duarte AC, Pereira E (2011) Immunosuppression in the infaunal bivalve Scrobicularia plana environmentally exposed to mercury and association with its accumulation. Chemosphere 82:1541–1546
Asokan R, Arumugam M, Mullainadhan P (1997) Activation of prophenoloxidase in the plasma and haemocytes of the marine mussel Perna viridis Linnaeus. Dev Comp Immunol 21:1–12
Bado-Nilles A, Gagnaire B, Thomas-Guyon H, Le Floch S, Renault T (2008) Effects of 16 pure hydrocarbons and two oils on haemocyte and haemolymphatic parameters in the Pacific oyster, Crassostrea gigas (Thunberg). Toxicol Vitro 22:1610–1617
Bado-Nilles A, Quentel C, Auffret M, Le Floch S, Gagnaire B, Renault T, Thomas-Guyon H (2009) Immune effects of HFO on European sea bass, Dicentrarchus labrax, and Pacific oyster, Crassostrea gigas. Ecotoxicol Environ Saf 72:1446–1454
Bado-Nilles A, Renault T, Faury N, Le Floch S, Quentel C, Auffret M, Thomas-Guyon H (2010) In vivo effects of LCO soluble fraction on immune-related functions and gene transcription in the Pacific oyster, Crassostrea gigas (Thunberg). Aquat Toxicol 97:196–203
Banni M, Bouraoui Z, Ghedira J, Clearandeau C, Jebali J, Boussetta H (2009) Seasonal variation of oxidative stress biomarkers in clams Ruditapes decussatus sampled from Tunisian coastal areas. Environ Monit Assess 155:119–128
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal Biochem 72:248–255
Breitwieser M, Thomas-Guyon H, Huet V, Sagerup K, Geraudie P (2018) Spatial and temporal impacts of the Skjervøy harbour diesel spill on native population of blue mussels: a sub-arctic case study. Ecotoxicol Environ Saf 153:168–174
Chalghmi H, Bourdineaud JP, Haouas Z, Gourves PY, Zrafi I, Saidane-Mosbahi D (2016a) Transcriptomic, biochemical, and histopathological responses of the clam Ruditapes decussatus from a metal-contaminated tunis lagoon. Arch Environ Contam Toxicol 70:241–256
Chalghmi H, Zrafi I, Gourves PY, Bourdineaud JP, Saidane-Mosbahi D (2016b) Combined effects of metal contamination and abiotic parameters on biomarker responses in clam Ruditapes decussatus gills: an integrated approach in biomonitoring of Tunis lagoon. Environ Sci Process Impacts 18:895–907
Chalghmi H, Zrafi I, Saidane-Mosbahi D (2016c) Chronic effects of petroleum hydrocarbons in Tunis-navigation channel on phase I and II biotransformation enzymes in bivalve species. Int J Res Chem Environ 6:28–33
Chalghmi H, Bourdineaud J-P, Chbani I, Haouas Z, Bouzid S, Er-Raioui H, Saidane-Mosbahi D (2020) Occurrence, sources and effects of polycyclic aromatic hydrocarbons in the Tunis lagoon, Tunisia: an integrated approach using multi-level biological responses in Ruditapes decussatus. Environ Sci Pollut Res 27:3661–3674
Cheng TC, Rodrick GE, Foley DA, Koehler SA (1975) Release of lysozyme from hemolymph cells of Mercenaria mercenaria during phagocytosis. J Invertebr Pathol 25:261–265
Coles JA, Farley SR, Pipe RK (1994) Effects of fluoranthene on the immunocompetence of the common marine mussel, Mytilus edulis. Aquat Toxicol 30:367–379
Cotou E, Tsangaris C, Henry M (2013) Comparative study of biochemical and immunological biomarkers in three marine bivalves exposed at a polluted site. Environ Sci Pollut Res 20:1812–1822
Croxton AN, Wikfors GH, Schulterbrandt-Gragg RD (2012) Immunomodulation in eastern oysters, Crassostrea virginica, exposed to a PAH-contaminated, microphytobenthic diatom. Aquat Toxicol 118–119:27–36. https://doi.org/10.1016/j.aquatox.2012.02.023
Díaz-Resendiz K, Romero-Bañuelos C, Robledo-Marenco M, Rojas-García A, Barrón-Vibanco B, Medina-Díaz I, Girón-Pérez M (2014) Deregulation of the humoral immune response of the oyster (Crassostrea corteziensis) exposed to naphthalene. Invert Surviv J 11:30–38
Duchemin MB, Auffret M, Wessel N, Fortier M, Morin Y, Pellerin J, Fournier M (2008) Multiple experimental approaches of immunotoxic effects of mercury chloride in the blue mussel Mytilus edulis, through in vivo, in tubo and in vitro exposures. Environ Pollut 153:416–423
Dyrynda EA, Law RJ, Dyrynda PE, Kelly CA, Pipe RK, Ratcliffe NA (2000) Changes in immune parameters of natural mussel Mytilus edulis populations following a major oil spill (Sea Empress, Wales, UK). Mar Ecol Prog Ser 206:155–170
Er-Raioui H, Bouzid S, Marhraoui M, Saliot A (2009) Hydrocarbon pollution of the Mediterranean coastline of Morocco. Ocean Coast Manag 52:124–129
Esposito G et al (2018) The bivalve Ruditapes decussatus: a biomonitor of trace elements pollution in Sardinian coastal lagoons (Italy). Environ Pollut 242:1720–1728
Fathallah S, Medhioub MN, Kraiem MM (2013) Sediment contact assay using the carpet shell clam, Ruditapes decussatus L., embryos and larvae for assessing contamination levels of four sites in Tunisian coast. Bull Environ Contam Toxicol 90:611–615
Gagnaire B, Frouin H, Moreau K, Thomas-Guyon H, Renault T (2006) Effects of temperature and salinity on haemocyte activities of the Pacific oyster, Crassostrea gigas (Thunberg). Fish Shellfish Immunol 20:536–547. https://doi.org/10.1016/j.fsi.2005.07.003
Guardiola FA, Cuesta A, Arizcun M, Meseguer J, Esteban MA (2014) Comparative skin mucus and serum humoral defence mechanisms in the teleost gilthead seabream (Sparus aurata). Fish. Shellfish. Immunol. 36:545–551
Haberkorn H et al (2014) Cellular and biochemical responses of the oyster Crassostrea gigas to controlled exposures to metals and Alexandrium minutum. Aquat Toxicol 147:158–167. https://doi.org/10.1016/j.aquatox.2013.12.012
Hannam ML, Bamber SD, Moody JA, Galloway TS, Jones MB (2009) Immune function in the Arctic Scallop, Chlamys islandica, following dispersed oil exposure. Aquat Toxicol 92:187–194. https://doi.org/10.1016/j.aquatox.2009.01.010
Hannam ML, Bamber SD, Galloway TS, John Moody A, Jones MB (2010a) Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. Chemosphere 78:779–784. https://doi.org/10.1016/j.chemosphere.2009.12.049
Hannam ML, Bamber SD, Moody AJ, Galloway TS, Jones MB (2010b) Immunotoxicity and oxidative stress in the Arctic scallop Chlamys islandica: effects of acute oil exposure. Ecotoxicol Environ Saf 73:1440–1448. https://doi.org/10.1016/j.ecoenv.2010.06.012
Hellal MEA, Hellal F, El Khemissi Z, Jebali R, Dachraoui M (2011) Trace metals in algae and sediments from the north-eastern Tunisian lagoons. Bull Environ Cont Toxicol 86:194–198
Hurtado MÁ, Da Silva PM, Le Goïc N, Palacios E, Soudant P (2011) Effect of acclimatization on hemocyte functional characteristics of the Pacific oyster (Crassostrea gigas) and carpet shell clam (Ruditapes decussatus). Fish Shellfish Immunol 31:978–984
Jouini Z, Charrada RB, Moussa M (2005) Caractéristiques du Lac Sud de Tunis après sa restauration Mar. Life 15:3–11
Khedir-Ghenim Z, Zrafi-Nouira I, Bahri R, Belayouni H, Hammami M, Rouabhia M, Saidane-Mosbahi D (2009) Identification and distribution of petroleum hydrocarbons in sediments, seawater and Ruditapes decussatus collected from a Mediterranean Sea site. Int J Water 5:35–50
Luna-Acosta A, Bustamante P, Godefroy J, Fruitier-Arnaudin I, Thomas-Guyon H (2010) Seasonal variation of pollution biomarkers to assess the impact on the health status of juvenile Pacific oysters Crassostrea gigas exposed in situ. Environ Sci Pollut Res 17:999–1008
Manoli E, Samara C (2008) The removal of polycyclic aromatic hydrocarbons in the wastewater treatment process: experimental calculations and model predictions. Environ Pollut 151:477–485. https://doi.org/10.1016/j.envpol.2007.04.009
Mansour C, Guardiola FA, Esteban MÁ, Mosbahi DS (2017) Combination of polycyclic aromatic hydrocarbons and temperature exposure: In vitro effects on immune response of European clam (Ruditapes decussatus). Fish Shellfish Immunol 67:110–118
Matozzo V, Binelli A, Parolini M, Previato M, Masiero L, Finos L, Bressan M, Marin MG (2012) Biomarker responses in the clam Ruditapes philippinarum and contamination levels in sediments from seaward and landward sites in the Lagoon of Venice. Ecol Ind 19:191–205
Matozzo V, Giacomazzo M, Finos L, Marin MG, Bargelloni L, Milan M (2013) Can ecological history influence immunomarker responses and antioxidant enzyme activities in bivalves that have been experimentally exposed to contaminants? A new subject for discussion in “eco-immunology” studies. Fish Shellfish Immunol 35:126–135
Mzoughi N, Chouba L (2011) Distribution of trace metals, aliphatic hydrocarbons and polycyclic aromatic hydrocarbons in sediment cores from the Sicily Channel and the Gulf of Tunis (south-western Mediterranean Sea). Environ Tec 32:43–54
Nasci C, Da Ros L, Campesan G, Van Vleet ES, Salizzato M, Sperni L, Pavoni B (1999) Clam transplantation and stress-related biomarkers as useful tools for assessing water quality in coastal environments. Mar Pollut Bull 39:255–260. https://doi.org/10.1016/S0025-326X(99)00094-6
Parry RM Jr, Chandan RC, Shahani KM (1965) A rapide and sensitive assay of muramidase. Proc Soc Exp Biol Med 119:384–386
Pretti C, Cognetti-Varriale AM (2001) The use of biomarkers in aquatic biomonitoring: the example of esterases. Aquat Conserv 11:299–303
Reynaud S, Deschaux P (2006) The effects of polycyclic aromatic hydrocarbons on the immune system of fish: a review. Aquat Toxicol 77:229–238
Romeo M, Hoarau P, Garello G, Gnassia-Barelli M, Girard JP (2003) Mussel transplantation and biomarkers as useful tools for assessing water quality in the NW Mediterranean. Environ Pollut 122:369–378
Sandrini-Neto L, Pereira L, Martins CC, de Assis HCS, Camus L, Lana PC (2016) Antioxidant responses in estuarine invertebrates exposed to repeated oil spills: effects of frequency and dosage in a field manipulative experiment. Aquat Toxicol 177:237–249
Solé M (2000) Assessment of the results of chemical analyses combined with the biological effects of organic pollution on mussels. TrAC Trends Anal Chem 19:1–9
Turja R, Soirinsuo A, Budzinski H, Devier MH, Lehtonen KK (2013) Biomarker responses and accumulation of hazardous substances in mussels (Mytilus trossulus) transplanted along a pollution gradient close to an oil terminal in the Gulf of Finland (Baltic Sea). Comp Biochem Physiol C 157:80–92. https://doi.org/10.1016/j.cbpc.2012.09.006
Velez C, Figueira E, Soares AMVM, Freitas R (2017) Effects of seawater temperature increase on economically relevant native and introduced clam species. Mar Environ Res 123:62–70. https://doi.org/10.1016/j.marenvres.2016.11.010
Walker CH, Hopkin SP, Sibly RM, Peakall DB (2001) Principles of Ecotoxicology, 2nd edn. Taylor & Francis, London
Wootton E, Dyrynda E, Pipe R, Ratcliffe N (2003) Comparisons of PAH-induced immunomodulation in three bivalve molluscs. Aquat Toxicol 65:13–25
Xie J, Zhao C, Han Q, Zhou H, Li Q, Diao X (2017) Effects of pyrene exposure on immune response and oxidative stress in the pearl oyster Pinctada martensii. Fish Shellfish Immunol 63:237–244
Zrafi-Nouira I, Khedir-Ghenim Z, Zrafi F, Bahri R, Cheraeif I, Rouabhia M, Saidane-Mosbahi D (2008) Hydrocarbon pollution in the sediment from the Jarzouna-Bizerte coastal area of Tunisia (Mediterranean Sea). Bull Environ Contam Toxicol 80:566–572
Acknowledgements
This study was supported by the Ministry of Scientific Research and Technology and the University of Monastir, Tunisia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Additional information
Communicated by Settimio Ferlisi, Chief Editor.
Rights and permissions
About this article
Cite this article
Mansour, C., Taheur, F.B., Omrani, R. et al. Immune biomarker and hydrocarbon concentrations in carpet shell clams (Ruditapes decussatus) collected from a Mediterranean coastal lagoon. Euro-Mediterr J Environ Integr 5, 11 (2020). https://doi.org/10.1007/s41207-020-0147-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-020-0147-4