Abstract
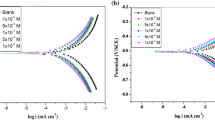
Inhibitive performance of 3-methylquinoxaline-2 (1H) -thione denoted (MQ = S) on mild steel corrosion in 1.0 M H3PO4 was investigated using electrochemical measurements. It is revealed that this product acts as an excellent corrosion inhibitor for mild steel in 1.0 M H3PO4 where its inhibition reaches 93% at 5 × 10−4 M and increases with immersion time. Potentiodynamic polarization curves indicated that this compound acts as a mixed-type inhibitor. In addition, the MQ = S has its inhibition at high temperature. It was found that the adsorption of this compound obeyed the Langmuir adsorption isotherm.











Similar content being viewed by others
References
Adardour K, Touir R, Elbakri M, Ramli Y, Touhami ME, El Kafsaoui H, Kalonji Mubengayi C, Essassi EM (2013) Thermodynamic study of mild steel corrosion in hydrochloric acid by new class synthesized quinoxaline derivatives: part II. Res Chem Intermed 39:4175–4188
Aramaki K, Hagiwara M, Nishihara H (1987) Adsorption and corrosion inhibition effect of anions plus an organic cation on iron in 1 M HClO4 and the HSAB principle. J Electrochem Soc 134:1896–1901
Bammou L, Belkhaouda M, Salghi R, Benali O, Zarrouk A, Zarrok H, Hammouti B (2014) Corrosion inhibition of steel in sulfuric acidic solution by the chenopodium ambrosioides extracts. J Assoc Arab Univ Basic Appl Sci 16:83–90
Benabdellah M, Benkaddour M, Hammouti B, Bendahhou M, Aouniti A (2006) Inhibition of steel corrosion in 2 M H3PO4 by artemisia oil. Appl Surf Sci 252:6212–6217
Benbouya K, Zerga B, Sfaira M, Taleb M, Ebn Touhami M, Hammouti B, Benzeid H, Essassi EM (2012) WL, IE and EIS studies on the corrosion behaviour of mild steel by 7-substituted 3-methylquinoxalin-2 (1H)-ones and thiones in hydrochloric acid medium. Int J Electrochem Sci 7:6313–6330
Benbouya K, Forsal I, Elbakri M, Anik T, Touir R, Bennajah M, Chebab D, Rochdi A, Mernari B, Ebn Touhami M (2014) Influence of pyridazine derivative on corrosion inhibition of mild steel in acidic media. Res Chem Intermed 40:1267–1281
Berthier F, Diard JP, Michel R (2001) Distinguishability of equivalent circuits containing CPEs: part I. Theoretical part. J Electroanal Chem 510:1–11
Bommersbach P, Alemany-Dumont C, Millet JP, Normand B (2005) Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical methods. Electrochim Acta 51:1076–1084
Bouckamp A (1993) Users manual equivalent circuit, ver. 4.51
Bozorg M, Shahrabi Farahani T, Neshati J, Chaghazardi Z, Mohammadi Ziarani G (2014) Myrtus communis as green inhibitor of copper corrosion in sulfuric acid. Indus Eng Chem Res 53:4295–4303
Brandt H, Fischer M, Schwabe K (1970) Untersuchungen über die sparbeizwirkungorganischer sulfide im system eisenschwefelsäure. Corros Sci 10:631–639
Brown DJ, Taylor EC, Ellman JA (2004) Heterocyclic compounds: the Quinoxalines: supplement II, vol 61. John Wiley, Hoboken, New Jersey, p 443
Brug GJ, Van Den Eeden ALG, Sluyters-Rehbach M, Sluyters JH (1984) The analysis of electrode impedances complicated by the presence of a constant phase element. J Electroanal Chem Interfacial Electrochem 176:275–295
Caliskan N, Akbas E (2011) The inhibition effect of some pyrimidine derivatives on austenitic stainless steel in acidic media. Mater Chem Phys 126:983–988
Cheeseman GWH, Cookson RF (1979) Condensed pyrazines. In: Cheeseman A, Taylor EC (eds) The chemistry of heterocyclic compounds, 35th edn. John Wiley, New Jersey
Chitra S, Parameswari K, Vidhya M, Kalishwari M, Selvaraj A (2011) Evaluation of quinoxalines as corrosion inhibitors for mild steel in acid environment. Int J Electrochem Sci 6:4593–4613
Donnelly B, Downie TC, Grzekowiak R, Hamburg HR, Short D (1978) The effect of electronic delocalization in organic groups R in substituted thiocarbamoyl RCSNH2 and related compounds on inhibition efficiency. Corros Sci 18:109–116
El Ouali I, Hammouti B, Aouniti A, Ramli Y, Azougagh M, Essassi EM, Bouachrine M (2010) Thermodynamic characterisation of steel corrosion in HCl in the presence of 2-phenylthieno (3, 2-b) quinoxaline. J Mater Environ Sci 1:1–8
Faustin M, Lebrini M, Robert F, Roos C (2011) Corrosion studies of C38 steel by alkaloids extract of a tropical plant type. Int J Electrochem Sci 6:4095–4113
Fiaud C, Harch A, Mallouh D, Tzinmann M (1993) The inhibition of iron corrosion by acetylenic alcohols in acid solutions at high temperature. Corros Sci 35:1437–1444
Georges C, Rocca E, Steinmetz P (2008) Synergistic effect of tolutriazol and sodium carboxylates on zinc corrosion in atmospheric conditions. Electrochimi Acta 53:4839–4845
Ghanbari A, Attar MM, Mahdavian M (2010) Corrosion inhibition performance of three imidazole derivatives on mild steel in 1 M phosphoric acid. Mater Chem Phys 124:1205–1209
Hegazy MA (2015) Novel cationic surfactant based on triazole as a corrosion inhibitor for carbon steel in phosphoric acid produced by dihydrate wet process. J Mol Liq 208:227–236
Hong S, Chen W, Zhang Y, Luo HQ, Li M, Li NB (2013) Investigation of the inhibition effect of trithiocyanuric acid on corrosion of copper in 3.0 wt% NaCl. Corros Sci 66:308–314
Ivanov ES (1986) Inhibitors for metal corrosion in acid media, metallurgy, Moscow
Jorcin JB (2007) Thesis, INP, France. http://ethesis.inp-toulouse.fr/archive/00000494/
Khaled KF, Al-Qahtani MM (2009) The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: chemical, electrochemical and theoretical studies. Mater Chem Phys 113:150–158
Kliškic M, Radoševic J, Gudic S (1997) Pyridine and its derivatives as inhibitors of aluminium corrosion in chloride solution. J Appl Electrochem 27:947–952
Lagrenee M, Mernari B, Bouanis M, Traisnel M, Bentiss F (2002) Study of the mechanism and inhibiting efficiency of 3, 5-bis (4-methylthiophenyl)-4H-1, 2, 4-triazole on mild steel corrosion in acidic media. Corros Sci 44:573–588
Leçe HD, Emregül KC, Atakol O (2008) Difference in the inhibitive effect of some Schiff base compounds containing oxygen, nitrogen and sulfur donors. Corros Sci 50:1460–1768
Li W, Hu L, Zhang S, Hou B (2011) Effects of two fungicides on the corrosion resistance of copper in 3.5% NaCl solution under various conditions. Corros Sci 53:735–745
Martinez S, Stern I (2002) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl Surf Sci 199:83–89
Menon P, Gopal M, Prasad R (2004) Influence of two insecticides, chlorpyrifos and quinalphos, on arginine ammonification and mineralizable nitrogen in two tropical soil types. J Agric Food Chem 52:7370–7376
Mernari B, El Attari H, Traisnel M, Bentiss F, Lagrenee M (1998) Inhibiting effects of 3, 5-bis (n-pyridyl)-4-amino-1, 2, 4-triazoles on the corrosion for mild steel in 1 M HCl medium. Corros Sci 40:391–399. doi:10.1016/S0010-938X(97)00142-X
Musa AY, Mohamad AB, Kadhum AAH, Takriff MS, Tien LT (2011) Synergistic effect of potassium iodide with phthalazone on the corrosion inhibition of mild steel in 1.0 M HCl. Corros Sci 53:3672–3677. doi:10.1016/j.corsci.2011.07.010
Obot IB, Obi-Egbedi NO, Odozi NW (2010) Acenaphtho [1, 2-b] quinoxaline as a novel corrosion inhibitor for mild steel in 0.5 M H2SO4. Corros Sci 52:923–926
Olasunkanmi LO, Kabanda MM, Ebenso EE (2016) Quinoxaline derivatives as corrosion inhibitors for mild steel in hydrochloric acid medium: electrochemical and quantum chemical studies. Phys E Low Dimens Syst Nanostruct 76:109–126
Rochdi A, Kassou O, Dkhireche N, Touir R, El Bakri M, Ebn Touhami M, Sfaira M, Mernari B, Hammouti B (2014) Inhibitive properties of 2, 5-bis (n-methylphenyl)-1, 3, 4-oxadiazole and biocide on corrosion, biocorrosion and scaling controls of brass in simulated cooling water. Corros Sci 80:442–452
Rochdi A, Touir R, El Bakri M, Touhami ME, Bakkali S, Mernari B (2015) Protection of low carbon steel by oxadiazole derivatives and biocide against corrosion in simulated cooling water system. J Environ Chem Eng 3:233–242
Sagüés AA, Kranc SC, Moreno EI (1996) Evaluation of electrochemical impedance with constant phase angle component from the galvanostatic step response of steel in concrete. Electrochim Acta 41:1239–1243
Sankarapapavinasam S, Pushpanaden F, Ahmed MF (1991) Piperidine, piperidones and tetrahydrothiopyrones as inhibitors for the corrosion of copper in H2SO4. Corros Sci 32:193–203
Satapathy AK, Gunasekaran G, Sahoo SC, Kumar A, Rodrigues PV (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci 51:2848–2856
Singh DDN, Chaudhary RS, Prakash B, Agarwal CV (1979) Inhibitive efficiency of some substituted thioureas for the corrosion of aluminium in nitric acid. Br Corros J 14:235–239
Stern M, Geary AL (1957) Electrochemical polarization I. A theoretical analysis of the shape of polarization curves. J Electrochem Soc 104:56–63
Tazouti A, Galai M, Touir R, Touhami ME, Zarrouk A, Ramli Y, Saraçoglu M, Kaya S, Kanderimili F, Kaya C (2016) Experimental and theoretical studies for mild steel corrosion inhibition in 1.0 M HCl by three new quinoxalinone derivatives. J Mol Liq 221:815–832
Thomas KJ, Tyagi P (2010) Synthesis, spectra, and theoretical investigations of the triarylamines based on 6 H-indolo [2, 3-b] quinoxaline. J Org Chem 75:8100–8111
Tian H, Li W, Hou B (2013) Electrochemical and theoretical investigation of triazole derivatives as corrosion inhibitors for copper in 3.5% NaCl solution. Int J Electrochem Sci 8:8513–8529
Touir R, Cenoui M, El Bakri M, Ebn Touhami M (2008) Sodium gluconate as corrosion and scale inhibitor of ordinary steel in simulated cooling water. Corros Sci 50:1530–1537
Touir R, Ebn Touhami M, Lakhrissi B (2014) Mild steel corrosion inhibition in 200 ppm NaCl by new surfactant. LAP LAMBRT Acadimic Publishing, Saarbrucken
Verma C, Singh P, Quraishi MA (2015) A thermodynamical, electrochemical and surface investigation of bis (indolyl) methanes as green corrosion inhibitors for mild steel in 1 M hydrochloric acid solution. JAAUBAS. doi:10.1016/j.jaubas.2015.04.003
Wang D, Xiang B, Liang Y, Song S, Liu C (2014) Corrosion control of copper in 3.5 wt% NaCl solution by domperidone: experimental and theoretical study. Corros Sci 85:77–86
Zarrok H, Zarrouk A, Salghi R, Hammouti B, Chahboun N, Ben Hmamou D, Hmammouchi R, Lakhlifi T, Rochdi A, El Assyry A (2014) Inhibition of carbon steel corrosion in HCL media by lipid oil melia. Mor J Chem 2:10–21
Zarrouk A, Hammouti B, Touzani R, Al-Deyab SS, Zertoubi M, Dafali A, Elkadiri S (2011) Comparative study of new quinoxaline derivatives towards corrosion of copper in nitric acid. Int J Electrochem Sci 6:4939–4952
Acknowledgements
The authors appreciate the experimental support given by all members of ‘Laboratoire d’Ingénierie des Matériaux et d’Environnement: Modélisation et Application, Faculté des Sciences, Université Ibn Tofail’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors have declared no conflicts of interest.
Rights and permissions
About this article
Cite this article
Benbouya, K., Dkhireche, N., Rochdi, A. et al. Adsorption and inhibitive performance of 3-methylquinoxaline-2(1H)-thione against mild steel corrosion in phosphoric acid solution. Euro-Mediterr J Environ Integr 3, 2 (2018). https://doi.org/10.1007/s41207-017-0042-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-017-0042-9