Abstract
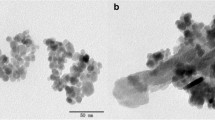
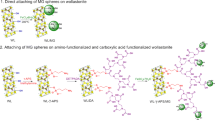
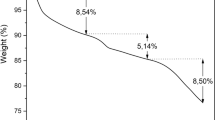
Magnetic adsorption is considered a promising technology for water treatment and solid–liquid separation. Porous and magnetic Fe3O4-hydroxyapatite (wFeHAp) nanocomposites were prepared from a Moroccan natural phosphate in the presence of Fe3O4 nanoparticles prepared by a controlled precipitation method. Their adsorption efficiency for the removal of trace metals was tested. After water treatment, separation of the wFeHAp powder from water was carried out by applying an external magnetic field. Thanks to their porosity for liquid–solid separation and their magnetic character depending on the Fe3O4 content, wFeHAp nanocomposites favorably immobilized the selected metals.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not Applicable.
References
Lu AH, Schmidt W, Matoussevitch N, Bönnemann H, Spliethoff B, Tesche B, Bill E, Kiefer W, Schüth F (2004) Nanoengineering of a magnetically separable hydrogenation catalyst. Angew Chem Int Ed 43:4303–4306
El Bekkali C, Labrag J, Oulguidoum A, Chamkhi I, Laghzizil A, Nunzi JM, Robert D, Aurag J (2022) Porous ZnO/hydroxyapatite nanomaterials with effective photocatalytic and antibacterial activities for the degradation of antibiotics. Nanotechnol Environ Eng 7:333–341
He L, WangM GJ, Yin Y (2012) Magnetic assembly route to colloidal responsive photonic nanostructures. Acc Chem Res 45:1431–1440
Gleich B, Weizenecker J (2005) Tomographic imaging using the nonlinear response of magnetic particles. Nature 435:1214–1217
Philip J, Shima PD, Raj B (2008) Nanofluid with tunable thermal properties. Appl Phys Lett 92:10–13
Philip J, Jaykumar T, Kalyanasundaram P, Raj B (2001) A tunable optical filter. Meas Sci Technol 14:1289–1294
Elliott DW, Zhang WX (2001) Field assessment of nanoscale bimetallic particles for groundwater treatment. Environ Sci Technol 35:4922–4926
GeorgouvelasvD AHN, Li J, Edlund U, Mathew AP (2021) All-cellulose functional membranes for water treatment: adsorption of metal ions and catalytic decolorization of dyes. Carbohydr Polym 264:118044
Bhaisare ML, Abdelhamid HN, Wu BS, Wu HF (2014) Rapid and direct MALDI-MS identification of pathogenic bacteria from blood via ionic liquid-modified magnetic nanoparticles (Fe3O4@SiO2). J Mater Chem B 2:4671–4683
Abdelhamid HN, Wu HF (2014) Facile synthesis of nano silver ferrite (AgFeO2) modified with chitosan applied for biothiol separation. Mater Sci Eng C Mater Biol Appl 45:438–445
Coulon A, Grandjean A, Laurencin D, Jollivet PK, Rossignol SE, Campayo L (2017) Durability testing of an iodate-substituted hydroxyapatite designed for the conditioning of 129I. J Nucl Mater 484:324–331
Yang K, Wang Y, Shen J, Scott SM, Riley BJ, Vienna JD, Lian J (2022) Cs3Bi2I9-hydroxyapatite composite waste forms for cesium and iodine immobilization. J Adv Ceram 11:712–728
Bouyarmane H, Asri El, Rami A, Roux C, Mahly MA, Saoiabi A, Coradin T, Laghzizil A (2010) Pyridine and phenol removal using natural and synthetic apatites as low cost sorbents: influence of porosity and surface interactions. J Hazard Mater 181:736–741
Oulguidoum A, Bouiahya K, Bouyarmane H, Talbaoui A, Nunzi JM, Laghzizil A (2021) Mesoporous nanocrystalline sulfonated hydroxyapatites enhance heavy metal removal and antimicrobial activity. Sep Purif Tech 255:117777
Ghate E, Ganjidoust H, Ayati B (2021) The thermodynamics, kinetics, and isotherms of sulfamethoxazole adsorption using magnetic activated carbon nanocomposite and its reusability potential. Nanotechnol Environ Eng 6:32
Astruc D, Lu F, Aranzaes JR (2005) Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 44:7852–7872
Tsang SC, Caps V, Paraskevas I, Chadwick D, Thompsett D (2004) Magnetically separable, carbon-supported nanocatalysts for the manufacture of fine chemicals. Angew Chem Int Ed 43:5645–5649
Shin S, Yoon H, Jang J (2008) Polymer-encapsulated iron oxide nanoparticles as highly efficient fenton catalysts. Catal Commun 10:178–182
Lei ZL, Li YL, Wei XY (2008) A facile two-step modifying process for preparation of poly(SStNa)-grafted Fe3O4/SiO2 particles. J Solid State Chem 181:480–486
Rehman GU, Tahir M, Goh PS, Ismail AF, Hafeez A, Khan IU (2021) Enhancing the photodegradation of phenol using Fe3O4/SiO2 binary nanocomposite mediated by silane agent. J Phys Chem Solids 153:110022
Rahimi SM, Panahi AH, Mazari Moghaddam NS, Allahyari E, Nasseh N (2022) Breaking down of low-biodegradation Acid Red 206 dye using Bentonite/Fe3O4/ZnO magnetic nanocomposite as a novel photo-catalyst in presence of UV light. Chem Phys Lett 794:139480
Watson S, Beydoun D, Amal R (2002) Synthesis of a novel magnetic photocatalyst by direct deposition of nanosized TiO2 crystals onto amagnetic core. J Photochem Photobiol A Chem 148:303–313
He QH, Zhang ZX, Xiong JW, Xiong YY, Xiao H (2008) A novel biomaterial-Fe3O4:TiO2 core–shell nanoparticle with magnetic performance and high visible light photocatalytic activity. Opt Mater 31:380–384
Mushtaq A, Zhao R, Luo D, Dempsey E, Wang X, Iqbal MZ, Kong X (2021) Magnetic hydroxyapatite nanocomposites: the advances from synthesis to biomedical applications. Mater Des 197:109269
Mondal S, Manivasagan P, Bharathiraja S, Moorthy MS, Kim HH, Seo H, Lee KD, Oh J (2017) Magnetic hydroxyapatite: a promising multifunctional platform for nanomedicine application. Int J Nanomed 12:8389–8410
Ansar EB, Ajeesh M, Yokogawa Y, Wunderlich W, Varma H (2012) Synthesis and characterization of iron oxide embedded hydroxyapatite bioceramics. J Am Ceram Soc 95:2695–2699
Foroutan R, Peighambardoust SJ, Ahmadi A, Akbari A, Farjadfard S, Ramavandi B (2021) Adsorption mercury, cobalt, and nickel with a reclaimable and magnetic composite of hydroxyapatite/Fe3O4/polydopamine. J Envir Chem Eng 9:105709
Vahdat A, Ghasemi B, Yousefpour M (2019) Synthesis of hydroxyapatite and hydroxyapatite/Fe3O4 nanocomposite for removal of heavy metals. Environ Nanotechnol Monit Manag 12:100233
Fan L, Liu H, Gao C, Zhu P (2022) Facile synthesis, and characterization of magnetic hydroxyapatite/ Fe3O4 microspheres. Mater Lett 313:131648
El Asri S, Laghzizil A, Saoiabi A, Alaoui A, El Abassi K, M’hamdi R, Coradin T, (2009) A novel process for the fabrication of nanoporous apatites from Moroccan phosphate rock. Colloid Surf A 350:73–78
Oulguidoum A, Bouiahya K, Bouyarmane H, Talbaoui A, Nunzi JM, Laghzizil A (2021) Mesoporous nanocrystalline sulfonated hydroxyapatites enhance heavy metal removal and antimicrobial activity. Sep Purif Technol 255:117777
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ahmed SM, Taha MR, Taha OME (2018) Kinetics and isotherms of dichlorodiphenyltrichloroethane (DDT) adsorption using soil–zeolite mixture. Nanotechnol Environ Eng 3:4
El Messaoudi N, El Khomri M, Chegini ZG, Bouich A, Dbik A, Bentahar S, Labjar N, Iqbal M, Jada A, Lacherai A (2021) Dye removal from aqueous solution using nanocomposite synthesized from oxalic acid-modified agricultural solid waste and ZnFe2O4 nanoparticles. Nanotechnol Environ Eng. https://doi.org/10.1007/s41204-021-00173-6
Kosmulski M (2019) The pH dependent surface charging and points of zero charge. VIII. Update Adv Colloid Interf Sci 275:102064
El Asri S, Laghzizil A, Coradin T, Saoiabi A, Alaoui A, M’hamedi R (2010) Conversion of natural phosphate rock into mesoporous hydroxyapatite for heavy metals removal from aqueous solution. Colloid Surf A 362:33–38
Ivanets AI, Kitikova NV, Shashkova IL, Roshchina MY, Srivastava V, Sillanpää M (2019) Adsorption performance of hydroxyapatite with different crystalline and porous structure towards metal ions in multicomponent solution. J Water Process Eng 32:100963
Ain QL, Farooq MU, Jalees MI (2020) Application of magnetic graphene oxide for water purification: heavy metals removal and disinfection. J Water Process Eng 33:101044
Zeng X, Zhang G, Zhu J (2022) Selective adsorption of heavy metals from water by a hyper-branched magnetic composite material: Characterization, performance, and mechanism. J Environ Manag 314:114979
Hu Z, Qin S, Huang Z, Zhu Y, Xi L, Li Z (2017) Stepwise synthesis of graphene oxide-wrapped magnetic composite and its application for the removal of Pb (II). Arab J Sci Eng 42:4239–4247
Maneechakr P, Mongkollertlop S (2020) Investigation on adsorption behaviors of heavy metal ions (Cd2+, Cr3+, Hg2+ and Pb2+) through low-cost/active manganese dioxide-modified magnetic biochar derived from palm kernel cake residue. J Envir Chem Eng 8:104467
Hosseini SS, Hamadi A, Foroutan R, Peighambardoust SJ, Ramavandi B (2020) Decontamination of Cd2+ and Pb2+ from aqueous solution using a magnetic nanocomposite of eggshell/starch/Fe3O4. J Water Process Eng 48:102911
Zhang X, Cheng T, Chen C, Wang L, Deng Q, Chen G, Ye C (2020) Synthesis of a novel magnetic nanozeolite and its application as an efficient heavy metal adsorbent. Mater Res Express 7:085007
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable. This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Consent to publication
Not applicable. This manuscript does not contain any individual person’s data in any form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Labrag, J., Abbadi, M., Oulguidoum, A. et al. Magnetic Fe3O4-Hydroxyapatite materials as adsorbents for the removal of metals from water. Nanotechnol. Environ. Eng. 8, 167–175 (2023). https://doi.org/10.1007/s41204-022-00292-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41204-022-00292-8