Abstract
The leather tanning industry is one of the important industrial activities in Egypt. However, this industry produces large amounts of wastewater containing trivalent chromium Cr (III) residues that are considered, if not treated, a significant risk to the environment. Although Cr (III) is not as toxic as Cr (VI), there is a high possibility that Cr (III) residues can be oxidized to Cr (VI). Due to this risk, chromium removal from tannery wastewater becomes a vital process. The current study demonstrates the ability of the use of different solid waste materials in the removal of trivalent chromium from tannery wastewater. Four agricultural waste materials and two industrial solid waste materials were tested. The agricultural solid waste materials include rice husk, rice straw, sugarcane bagasse and sawdust; while the industrial waste materials include cement kiln bypass dust and marble powder. The results showed that the cement kiln dust and marble powder can provide the highest removal of chromium from aqueous solution. The results also showed that cement kiln dust can remove more than 99% of the trivalent chromium when the pH of the solution is raised above 8.75.
Similar content being viewed by others
Introduction
The industrial development is an important part of the economic growth of any nation. However, industrial activities have serious environmental impacts including high water consumption and high polluted wastewater production. Globally, petrochemical, refinery, food, textile, pharmaceutical, and other industries are consuming huge amounts of freshwater which can be estimated to be 10% of the freshwater used in 2010 and this percentage is expected to increase to be 20% in 2025 [1]. Consequently, these industries produce huge amounts of heavily polluted wastewater that result in serious environmental and health problems. One of these industries is the leather industry. In Egypt, leather industry is an important industry that is considered as one of the backbone industries for the Egyptian economy. There are more than 300 tanneries that produce around 95 million square metres of tanned leather annually [2]. Despite its economic importance, this industry has very serious environmental impacts due to the hazardous solid and liquid wastes produced during the tanning process.
Tanning is the process of converting animal hides and skins into leather using tanning agents to give the leather its unique characteristics. Chrome tanning is the common type of tanning that is used in 90% of tanned hides around the world [3]. This process can produce highly polluted wastewater that is estimated to be 30–56 m3 per ton of hides or skin. This wastewater contains around 30–40% of the chromium used in the tanning process [2, 4, 5]. Most of chromium residues in the tanning wastewater are in the trivalent oxidation state.
Chromium is one of the heavy metals that have different oxidation states, however, it is stable in the trivalent and hexavalent oxidation states. Generally, chromium is an important industrial metal as it is used in many industries including metallurgical, chemical and refractory industries [6]. In the tanning industry, basic chromium sulphate salt (Cr2(SO4)3), which is a trivalent chromium salt, is the common tanning agent used in chrome tanning [7, 8]. Trivalent chromium (Cr (III)) is less in toxicity and solubility than hexavalent chromium (Cr(VI)) [9]. Certain concentrations of trivalent chromium are important for the metabolism process in humans and animals [10]. However, high concentrations of trivalent chromium can accumulate in animal and human tissues causing damage in the DNA [11]. Also, in alkaline and strong oxidation conditions, oxidants like peroxides and hypohalite can oxidize trivalent chromium into hexavalent chromium. These conditions can take place through the sterilization process of drinking water resulting in producing carcinogenic chromate and dichromate salts [12].
In Egypt, tannery wastewater treatment typically include screening, equalization, coagulation, flocculation, and sedimentation [13]. This treatment removes coarse matters, trivalent chromium Cr (III), sulphides and most of the suspended solids. However, other advanced treatment methods showed good performance in chromium removal like ion exchange, membrane filtration, adsorption, and chemical precipitation [5, 14,15,16].
The Egyptian Environmental Affairs Agency (EEAA) reported that the total amount of solid wastes in Egypt has increased from 2001 to 2012, by 24.26% to reach 89.28 million tons per year. It was also reported that, the agricultural solid wastes increased to become 33.6% of the total solid wastes generated in 2012 and the industrial solid wastes increased to become 6.72% of the total solid wastes generated in the same year [17]. The agricultural solid wastes generated include around five million tons per year of rice straw and one million tons per year of rice husk. Only a small amount of these types of agricultural wastes are used for animal feeding and the majority are burned in open fields or dumped as solid wastes [18, 19]. In addition, the agricultural sector in Egypt generated around 4.8 million tons of sugarcane bagasse annually from which around 14.4% is unutilized [18]. Some studies showed the possibilities of using different types of agricultural solid wastes, as low-cost adsorbents or coagulants for heavy metals removal [20,21,22]. It was reported that rice straw and rice husk showed good removal efficiency of heavy metals from aqueous solutions. A removal efficiency of 94% for lead and 100% for arsenic from aqueous solutions were achieved using rice straw and rice husk, respectively [23, 24]. Sugarcane bagasse could be used for nickel and lead removal from aqueous solutions. It has a good adsorption capacity that can be increased by chemical modification [25, 26]. Sawdust, another type of agricultural waste, could be used for the removal of heavy metals from aqueous solutions. Removal efficiencies of 100% for copper (II) and 94.61% for lead (II) were achieved using sawdust. It was also reported that it has a good adsorption capacity for Cr (VI) [27, 28].
On the other hand, industrial wastes such as cement kiln dust (CKD) and marble powder (MP) showed high potential for heavy metals removal from wastewater. Cement kiln dust is the micro-sized high alkali powder that is generated as a by-product from cement production process. It consists of a mixture of chemical compounds in which calcium oxide (CaO) is the major constituent and the other constituents include silicon dioxide (SiO2), aluminium oxide (Al2O3), ferric Oxide (Fe2O3), potassium oxide (K2O), sodium oxide (Na2O), chloride ions (Cl−) and other compounds [28,29,30]. The high contents of alkalis prevent the reuse of CKD in the cement manufacturing process; however, some research studies indicated that CKD can be reused in the cement production after removing the high contents of chlorine and potassium. Also, there are other beneficial uses of CKD such as replacement of cement in concrete mortar, soil stabilization and wastewater treatment [29, 30]. It was estimated that the cement industries in Egypt generate around 1 million tons of CKD annually [29]. Due to its alkalinity nature, cement kiln dust could be used to remove heavy metals from industrial wastewater. The cement bypass kiln dust leachate, which is a very alkaline solution (pH > 12), was used to completely precipitate copper (II), Nickel (II), and zinc (II) from synthetic solutions at pH values of 5.5, 7 and 7.3, respectively [27]. Also, it was used in tanneries wastewater treatment and the results showed significant reduction in the concentrations of chromium III, TSS and COD by 31, 92.1 and 91.3%, respectively [28]. Marble powder is another type of industrial wastes that has beneficial uses in producing concrete and high strength concrete [31]. Due to the availability and low cost of marble powder, many studies investigated its efficiency in heavy metals removal. Marble powder used for zinc (II) removal resulted in approximately 100% removal of zinc (II) [32]. In addition, the efficiency of using marble powder in copper (II) removal from drinking water was investigated and the results indicated that the removal of Cu2+ ions occurred mainly due to adsorption at pH < 6.0; however, at pH > 6.0, complete removal occurred due to both the concurrent precipitation of copper hydroxide (Cu (OH) 2(s)) and adsorption [33]. On the other hand, batch adsorption tests were conducted in order to study the efficiency of using marble powder in chromium (III) removal from tanneries wastewater and the results indicated that the maximum adsorption capacity was 262 mg/g at pH 5.0 and dose of 12 g/l of marble powder for initial concentration of chromium up to 3.21 g/l [34].
The current study aims at investigating utilization of different solid waste materials in the removal of trivalent chromium from synthetic chromium solution to select the waste material with the best performance. Also, it aims at identifying the removal mechanism of the selected material and compare its performance with other common chemical materials that were used in chromium removal. This study also aims at investigating the ability of the selected material to remove Cr (III) from real tannery`s wastewater and the effect of this material on other water quality parameters in addition to the economic benefit that can be reached by using this material in the conventional tannery`s wastewater treatment process. This study is very important as it shows the most efficient waste material that can be used in tannery`s wastewater treatment without adding additional cost or special preparations.
Materials and methods
Experimental setup and conditions
The experiments of the current study were conducted in three different experimental runs. In the first run, the ability of different solid waste materials to remove Cr (III) from aqueous solution was investigated. The waste materials used in this run were selected upon its availability in Egypt and its efficiency in heavy metals removal as discussed previously. The material of the best performance in Cr (III) removal was selected for further investigation in the following runs. In the second experimental run, the mechanism of chromium removal using the material selected from run 1 was identified. In the third experimental run, the performance of the selected solid waste material (from the first run) was tested on real tannery wastewater to identify the optimum dose for Cr (III) removal under real conditions.
Solid waste materials used in the experiment and their preparations
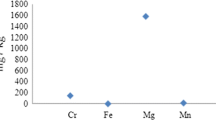
Two categories of solid waste materials were tested. They included agricultural solid wastes and industrial solid wastes. The agricultural solid wastes included rice straw (RS), rice husk (RH), sugarcane bagasse (SB) and sawdust (SD). The agricultural waste materials were prepared by washing successively with tap water for three times then rinsing with distilled water to remove any attached impurities. After that, they were dried in the sun for 24 h then in the oven at 80 °C for seven hours or till complete drying. Then, the dried materials were ground and sieved using a mechanical sieving system on a 100/200 sieve to obtain particle size range of 75–150 µm [2, 23, 35, 36]. The industrial solid wastes include marble powder (MP) and cement kiln dust (CKD). MP was ground and dried in an oven at 100 °C for 5 h then cooled. It was then sieved with Sieve No. 140 to obtain a more uniform particle size. The preparation of CKD followed similar process to MP excluding the initial grounding step. The chemical composition of CKD includes 55.1% CaO, 3.12% MgO, 3.33% Al2O3, 4.28% Fe2O3, 16.84% SiO2, 1.15% Na2O, 1.74% K2O, 3.22% SO3 and 3.43% Cl [37].
Preparation of the synthetic solution preparation
The synthetic solution of Cr (III) used in the experiments of the current study was prepared to have an initial concentration of 1000 mg Cr (III)/l. This solution was synthesized by dissolving 3.473 g of Cr (III) sulphate basic (Cr4(SO4)5(OH)2) in 500 ml of distilled water then the solution was completed to 1000 ml using distilled water. The pH of the solution was adjusted to 3.00 using a 4 M NaOH solution and 4 M HNO3 solution.
Real tannery wastewater
The real tannery wastewater that was used in the third experimental run was collected from one of the tanneries in El-Monofia Governorate, Egypt. The characteristics of the wastewater was analysed and it was found that it had pH of 3.63, 21.674 g/l Cr (III), 395 mg/l total suspended solids (TSS), 10.5 g/l chemical oxygen demand (COD), and 570 mg/l biochemical oxygen demand (BOD5). All these characteristics were reanalysed after treatment.
Experimental method
Experimental run 1: screening of the solid waste materials
In the first experimental run, seven high-density polyethylene bottles were used as the reactors. Each bottle was filled with 50 ml of Cr (III) synthetic solution with initial concentration of 1000 mg Cr (III)/l. Then 1 g of each type of the waste materials was added to each bottle except for the control (in which no material was added). After mixing well, the bottles were placed on a mechanical orbital shaker at speed of 200 RPM for 60 min. After that, the mixture in each bottle was allowed to settle for 20 min and then filtered using cellulose acetate filter paper (0.45 µm) and the filtrates were collected for the analysis of trivalent chromium. Chromium was analysed in the samples using flame atomic absorption spectrometer, Sensa AA dual-flame atomization system model, manufactured by GBC Scientific Equipment in USA.
Experimental run 2: mechanism of chromium removal using the best solid waste material
The experimental run was conducted to investigate the mechanism of chromium removal using the material of the best performance selected in the first experimental run. In addition, the investigated mechanism was compared to the mechanism of two chemical compounds which are commonly used for metal removal from aqueous solution. These compounds were magnesium oxide (MgO) and sodium hydroxide (NaOH).
The second experimental run was conducted in three batches using jar tester manufactured by Phipps & Bird, USA. In the first batch, six reactors (1 L jars) were filled with 500 ml of the synthetic chromium solution. Six different doses of the selected material, namely 0.5, 1, 3, 5, 10 and 20 g, were added to the reactors. Then, the contents of each reactor were flash mixed for 1 min at speed of 150 RPM followed by gentle mixing for 20 min at a speed of 40 RPM. The contents of each reactor were allowed to settle for 20 min and then filtered using cellulose acetate filter paper (0.45 µm). The filtrates were collected for the analysis of trivalent chromium using flame atomic absorption spectrometer. In the second batch, the same steps of the Jar Test were conducted using magnesium oxide (MgO) instead of the selected waste material. MgO was added to the synthetic chromium solution in doses similar to that of the selected waste material added in the first batch. In the third batch, five reactors were used, and sodium hydroxide was added instead of the selected waste material or MgO. The sodium hydroxide (1 M NaOH solution) was added to the synthetic chromium solutions in the reactors at doses of 2.1, 9.4, 12.5, 15.5 and 19.6 ml. These doses of NaOH were added to reach the same values of pH of the solutions treated by material of the best performance doses in the first batch.
Experimental run 3: testing of the best performance material on real tannery wastewater
The experiments of this run were conducted to investigate the removal of chromium from real tannery wastewater using the material of the best performance selected in the first experimental run. The real wastewater was collected from the tanning stage in one of the biggest tanneries in Egypt. Jar test was conducted to identify the optimum dose of the selected material for the removal of trivalent chromium from the real tannery wastewater. Seven doses of material of the best performance were tested. These doses included 3, 5, 10, 15, 20, 25 and 30 g of CKD per 500 ml of the tannery wastewater. The jar test was conducted by adding 500 ml of the real tannery wastewater into each jar then doses of the selected material was added, flash mixed for 1 min at a speed of 150 RPM, gentle mixed for 20 min at 40 RPM [37]. After mixing, the contents of the jars were allowed to settle for 20 min and then filtered using cellulose acetate filter paper 0.45 µm. The filtrates were collected for the analysis of trivalent chromium using flame atomic absorption spectrometry and the filtrate with the highest removal was analysed for TSS, COD and BOD5.
Results and discussion
Figure 1 shows the removal efficiencies of Cr III for the two groups of solid waste materials. As shown in the figure, the removal efficiency varies widely between the agricultural waste materials and the industrial waste materials. The efficiency ranged between 0.4 and 13.3% for the agricultural solid wastes. On the other hand, the efficiency for the industrial solid wastes reached 98.8% and 99.9% for MP and CKD, respectively.
Figure 2 shows the change in the pH value of the solution by the end of the experiments for different waste materials used for chromium removal. As shown in Fig. 2, the MP and CKD raised the pH value of the solution to 7.78 and 7.13, respectively, as compared to the pH value of the control (2.52). As shown also in Fig. 2, the pH values of the solutions after using the agricultural wastes were slightly higher than the control and ranged from 2.89 to 3.87. The results shown in Figs. 1 and 2 indicate that the high removal efficiency of Cr (III) using CKD and MP is attributed to the increase in the pH value of the solution. It is known that Cr (III) has a minimum solubility at pH range of 6.00–9.00 and precipitate as chromium hydroxide (Cr(OH)3) [38]. In addition, it was observed by the end of the experiments that the CKD and MP form clear solution on top of a greyish-green layer of particulate precipitate of chromium hydroxide (Cr (OH)3). The results obtained in Figs. 1 and 2 as well as the observations during the experiments suggest that the mechanism of chromium removal using industrial solid wastes involves chemical precipitation. This mechanism is different than the treatment mechanism of agricultural solid wastes which is based on adsorption mechanism [36, 39].
As CKD showed the highest removal of chromium (99.9%), therefore it was selected as a material of the best performance for testing in treating real tannery wastewater and for further investigation to understand its mechanism for Cr III removal. Magnesium Oxide (MgO) is commonly used as a coagulant and a metal precipitant in water and wastewater treatment. Therefore, the performance of CKD was compared with the performance of MgO in the removal of the trivalent chromium. Figure 3 shows the chromium removal efficiency at different doses of CKD and MgO. It shows that the performance of CKD and MgO were identical in the removal of the trivalent chromium. Figure 4 shows the change in the pH values at different doses of CKD and MgO. As shown in Fig. 4, identical change in the pH was obtained for CKD and MgO. From Figs. 3 and 4, it was noticed that the increase in the dose of CKD or MgO improve the Cr (III) removal efficiency. It was also noticed that the pH value increases with the increase in the removal efficiency of Cr (III) due to the increase in the CKD or MgO doses. Removal efficiencies above 99.9% can be obtained at a CKD or MgO doses above 2 g per litre of chromium solution. The resulting pH of the solution after treatment ranged from 8.75 to 12.34 at doses ranged from 2 g/L to 40 g/L, respectively, for both CKD and MgO. This pH range also supports the complete removal of Cr (III) from the solution. The increase in the pH after treatment can be attributed to the composition of the CKD, which include high contents of the following metal oxides: CaO, MgO, Al2O3 and Fe2O3 as shown in Table 1. These oxides can work as precipitants and coagulants when added to water. They form metal hydroxides which work as coagulants. The dissociation of the CKD in water also results in an increase in the hydroxyl ions causing the pH to rise and as a result formation of insoluble particulates of chromium. The metal hydroxide coagulants formed due to the addition of CKD remove the insoluble particulates of chromium from the solution and settle down. This results in a clarified solution on top of a greyish-green layer of particulate precipitate as observed in the experiments.
To investigate on the effect of raising pH on the removal of Cr III, sodium hydroxide (NaOH), a common base, was added at different doses. The added doses of sodium hydroxide raised the pH to values similar to those obtained after the addition of CKD to the chromium solution. Figure 5 shows a comparison of the effect of pH due to the addition of CKD and the addition of 1 M NaOH on the removal efficiency of the trivalent chromium.
As shown in Fig. 5, chromium removal of 85% was achieved at a pH value of 5.2 after the addition of CKD dose of 1 g/L. The increase in the CKD dose increased the pH of the solution and resulted in higher removal of chromium. However, the removal of chromium follows a different trend with the addition of NaOH. As shown in Fig. 5, there was no removal at pH 5.2 with the addition of NaOH. Then as the pH increased, the removal increased till it reaches the highest removal (95%) at pH 11.47. Then, the removal efficiency started to decrease with the increase in the pH beyond 11.47. In addition, it was observed during the experiments that the addition of NaOH resulted in the formation of suspended particulates. These particulates were kept in suspension and did not settle out easily from the solution. Sodium hydroxide dissociates in water resulting in the increase of the pH and as a result decrease in the solubility of Cr III and formation of chromium particulates. These particulates were kept in suspension as it requires coagulants to aid their removal and settling. Since Na + is a weaker coagulant compared to Ca+2, Mg+2, Al+3 and Fe+3 which are present in abundance in the CKD. Therefore, less removal was obtained using NaOH as compared with CKD as shown in Fig. 5.
Figure 6 shows the removal efficiency of trivalent chromium using CKD added to real tannery wastewater. Figure 6 also shows the change in the pH of the wastewater after treatment with different doses of the CKD. As shown in Fig. 6, the removal efficiency of Cr III increased by increasing the dose of CKD. The removal efficiency of Cr III ranged from 75 to 100% at CKD dose ranged from 6 g/L to 60 g/L. As shown in Fig. 6, the minimum CKD dose needed for the highest removal of chromium was 30 g/L or higher. It is also noticed from Fig. 6 that the minimum pH for the complete removal of Cr III is 8.75. This pH value occurs after the addition of CKD dose of 30–40 g/L. This pH value is also similar to that obtained for the complete removal of Cr III from the synthetic solution in the second experimental run.
The addition of CKD also improves the quality of tannery wastewater by the removal of other contaminants such as total suspended solids, chemical oxygen demand and 5-days biochemical oxygen demand as shown in Table 1. Removal efficiencies of 52.7%, 51.5% and 75.8% were achieved for TSS, COD, and BOD5, respectively. The ability of CKD to remove these pollutants is another indication that the CKD removal mechanism when added to wastewater involves coagulation and precipitation [40].
Application method of CKD in the conventional tannery’s wastewater treatment process
The current study shows that CKD can be used effectively in the removal of chromium from tannery wastewater. As a waste material, CKD is an economical replacement for precipitant/coagulants (such as MgO) that are commonly used in conventional tannery wastewater treatment. To replace commonly used coagulant, a simple modification in the process might be considered by using a dosing belt to add the CKD which is in a powder form. Also, to reduce inhalation risk of CKD fine particulates at the treatment, CKD can be added to the wastewater in liquid form by mixing the required doses of CKD with water before addition to tannery wastewater.
The sludge generated from conventional treatment due to the application of CKD can be thickened using gravity thickener or centrifugal cyclone then dewatered using filter press. Like the case in common precipitants, the sludge cake generated from the dewatering process can be treated for the recovery of chromium. Similar to common methods of chromium recovery from sludge of tannery industry, sulphuric acid (H2SO4) can be applied to the sludge cake to recover the trivalent chromium as chromium sulphate (Cr2(SO4)3) [41].
Economic benefits of using CKD in tannery`s wastewater treatment
Based on the results of the experiments of the current study, CKD can replace common precipitant/coagulants such as MgO in the conventional tannery wastewater treatment. The cost of MgO material, without including the cost of transportation, is 200,000 L.E./ Tonne (12,500 US $/ Tonne) in the Egyptian market. As the CKD is considered a solid waste material, it has no cost, except for transportation. As shown in the current study, both of CKD and MgO have identical performance in the removal of chromium from tannery wastewater. A dose of 50 g of MgO is needed for complete removal of Cr III from 1 L of tannery`s wastewater. Knowing that the tanning process of 1 tonne of hides can produce 56 m3 of wastewater that contains high chromium concentration (42). Based on the experiment of the current study, the required dose of MgO to completely remove chromium from this wastewater is 50 g/L. Thus, the total amount of MgO needed for the treatment of wastewater generated from the tanning of 1 Tonne of hides is 2.8 Tonne, which costs 560,000 L.E. (35,000 US $). Thus, replacing MgO with CKD can save this material cost and reserve the resources. On the other hand, the recovered chromium sulphate (Cr2(SO4)3) can replace around 30% of pure Cr2(SO4)3 that is used in the tanning process saving around 180,000 L.E/1 tonne of Cr2(SO4)3 (11,230 US $/Tonne) according to the Egyptian market [41]. During the tanning process of 1 Tonne of hides, around 130 kg of Cr2(SO4)3 is used [7, 42]. Thus, the reuse of the recovered Cr2(SO4)3 in leather tanning industry can save around 23,400 L.E (1,460 US $) per Tonne of hides.
Conclusion
Cr (III) removal is one of the important steps in tanning wastewater treatment. Agricultural and industrial solid wastes can be used as low-cost materials for Cr (III) removal. Comparing the removal efficiencies of two groups of solid wastes, it can be concluded that the industrial solid waste group, including marble powder and cement kiln dust, showed better removal efficiency than the agricultural solid waste group including rice straw, rice husk, sugarcane bagasse, and sawdust. The removal efficiencies of Cr (III) of the agricultural solid wastes group ranged from 0.4 to 13.3% and the removal efficiencies of the industrial solid wastes exceeded 98%. The high removal efficiencies of Cr (III) of the industrial solid wastes were combined with high pH values exceeded pH 6.00. Among the industrial waste materials, CKD is the best solid waste material recommended for Cr (III) removal from tannery wastewater. It can be removed completely through addition of CKD at the proper dose that can raise the pH of the wastewater above 8.75. For the tannery wastewater examined in the current study, a minimum CKD dose of 30 g/L is recommended for the complete removal of Cr III. Also, the CKD can significantly remove TSS, COD and BOD5 from the tannery wastewater. Removal efficiency of 52.7%, 51.5% and 75.8% can achieved for TSS, COD, and BOD, respectively. It was proven in the current study through comparison of performance with typical coagulant (MgO) and precipitant (NaOH) as well as visual observations, that the removal mechanism(s) of Cr III using CKD involve coagulation/precipitation.
Data availability
All data generated or analysed during this study are included in this published article.
References
Glavič P, Bogataj M (2011) Water networks–theory and practice. In: Atimtay A, Sikdar S (eds) Security of industrial water supply and management. NATO Science for Peace and Security Series C, Environmental Security, pp 13–30
Abdulla H, Ahmed E (2010), Chromium removal from tannery wastewater using chemical and biological techniques aiming zero discharge of pollution. Proceeding of fifth scientific environmental conference Zagazig University, 171–83.
Khan K, Khan I, Islam I, Mahmud A, Hossain D (2018) Recovery and reuse of chromium from spent chrome tanning liquor by precipitation process. Am J Eng Res 7(1):346–352
Ayaliew Werkneh A, Hayelom Dargo A, Ayalew A, (2014), Tannery waste water treatment: a review. Int J Emerg Trends Sci Technol. Available from: www.ijetst.in
El-Khateeb MA, Nashy E, Ghany N, Awad AM (2017) Environmental impact elimination of chrome tanning effluent using electrocoagulation process assisted by chemical oxidation. Desalin Water Treat 65:147–152
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2013) Heavy metals toxicity and the environment. NatlInst Health 101:133–164
Ramanujam RA, Ganesh R., Kandasamy J, (2010) Wastewater treatment technology for tanning industry. Encycl Life Support Syst.
Nazer DW, Al-SaEd RM, Siebel MA (2006) Reducing the environmental impact of the unhairing-liming process in the leather tanning industry. J Clean Prod 14:65–74
Rodríguez MC, Barsanti L, Passarelli V, Evangelista V, Conforti V, Gualtieri P (2007) Effects of chromium on photosynthetic and photoreceptive apparatus of the alga Chlamydomonasreinhardtii. Environ Res 105(2):234–239
Oliveira H (2012) Chromium as an environmental pollutant: insights on induced plant toxicity. J Bot 1–8.
Stearns DM, Belbruno JJ, Wetterhahn KE (1995) A prediction of chromium(III) accumulation in humans from chromium dietary supplements. FASEB J 9(15):1650–1657
Kolomaznik K, Adamek M, Andel I, Uhlirova M (2008) Leather waste-Potential threat to human health, and a new technology of its treatment. J Hazard Mater 160(2–3):514–520
Buljan J, Kral I (2011) Introduction to treatment of tannery effluents. United Nations Industrial Development Organization.
Fabbricino M, Gallo R (2010) Chromium removal from tannery wastewater using ground shrimp shells. Desalin Water Treat 23(1–3):194–198
Kocurek P, Kolomazník K, Barinova M (2015) Removal of chromium from wastewater by reverse osmosis. Russ J Phys Chem 89(7):1238–1243
Rengaraj S, Yeon KH, Moon SH (2001) Removal of chromium from water and wastewater by ion exchange resins. J Hazard Mater 87(1–3):273–287
Zaki T, Kafafi AG, Mina MB, Abd-El-Halim A, Saber M, (2013) Annual report for solid waste management in Egypt. 1–162. Retrived from: http://cairoclimatetalks.net/sites/default/files/EN Annual Report on Waste in Egypt_2013.pdf
Abdelhady S, Borello D, Shaban A, Rispoli F (2014) Viability study of biomass power plant fired with rice straw in Egypt. Energy Procedia 61, 211–5. Retrived from: https://doi.org/10.1016/j.egypro.2014.11.1072
Abo-El-Enein SA, Eissa MA, Diafullah AA, Rizk MA, Mohamed FM (2011) Utilization of a low cost agro-residue for production of coagulant aids and their applications. J Hazard Mater 186(2–3):1200–1205
Renu B, Agarwal M, Singh K (2017) Heavy metal removal from wastewater using various adsorbents: a review. J Water Reuse Desalin 7(4):387–419
Amer H, El-Gendy A, El-Haggar S (2017) Removal of lead (II) from aqueous solutions using rice straw. Water Sci Technol 76(5):1011–1021
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents-A critical review. J Hazard Mater 142(1–2):1–53
Aloma I, Martin-Lara MA, Rodriguez IL, Zquez GB, Calero M (2002) Removal of nickel (II) ions from aqueous solutions by biosorption on sugarcane bagasse. Proceedings of the ACM International Multimedia Conference and Exhibition.
Homagai PL, Ghimire KN, Inoue K (2010) Adsorption behavior of heavy metals onto chemically modified sugarcane bagasse. Biores Technol 101:2067–2069
Ahmad A, Rafatullah M, Sulaiman O, Ibrahim MH, Chii YY, Siddique BM (2009) Removal of Cu(II) and Pb(II) ions from aqueous solutions by adsorption on sawdust of Meranti wood. Desalination 247:636–646
Gupta S, Babu BV (2009) Removal of toxic metal Cr(VI) from aqueous solutions using sawdust as adsorbent: equilibrium, kinetics and regeneration studies. Chem Eng J 150:352–365
Zaki NG, Khattab IA, Abd El-Monem NM (2007) Removal of some heavy metals by CKD leachate. J Hazard Mater 147:21–27
Mostafa HM, Rashed M, Mostafa AH (2005), Utilization of by-pass kiln dust for treatment of tanneries effluent wastewater. Ninth International Water Technology Conference, IWTC9 2005.
Seo M, Lee SY, Lee C, Cho SS (2019) Recycling of cement kiln dust as a raw material for cement. Environments 6(10):113
Kunal A, Siddique R, Rajor A (2012) Use of cement kiln dust in cement concrete and its leachate characteristics. Resour Conserv Recycl 61:59–68
Alyousef R, Benjeddou O, Khadimallah MA, Mohamed AM, Soussi C (2018) Study of the effects of marble powder amount on the self-compacting concretes properties by microstructure analysis on cement-marble powder pastes. Adv Civil Eng.
Ghazy SE, Gad A (2008) Separation of Zn(II) by sorption onto powdered marble wastes. Indian J Chem Technol 15:433–442
Ghazy SE, Samra SE, Mahdy AM, El-Morsy SM (2003) Removal of copper(II) ions from Aqueous Solutions. I. adsorption studies using powdered marble wastes as sorbent. AdsorptSciTechnol 21:285–295
Elabbas S, Mandi L, Berrekhis F, Pons MN, Leclerc JP, Ouazzani N (2016) Removal of Cr(III) from chrome tanning wastewater by adsorption using two natural carbonaceous materials: eggshell and powdered marble. J Environ Manage 166:589–595
Mahmood-ul-Hassan M, Suthar V, Rafique E, Ahmad R, Yasin M (2015) Kinetics of cadmium, chromium, and lead sorption onto chemically modified sugarcane bagasse and wheat straw. Environ Monit Assess 187:470
Rocha CG, Zaia D, Alfaya R, Alfaya A (2009) Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J Hazard Mater 166:383–388
Abdelkader S (2019) Sustainable treatment of tanneries wastewater using low-cost and Highly efficient materials. Thesis, American University in Cairo. Retrieved from: http://dar.aucegypt.edu/handle/10526/5777
Unceta N, Séby F, Malherbe J, Donard O (2010) Chromium speciation in solid matrices and regulation: a review. Anal BioanalChem 397(3):1097–1111
Ding Y, Jing D, Gong H, Zhou L, Yang X (2012) Biosorption of aquatic cadmium(II) by unmodified rice straw. Biores Technol 114:20–25
Minas F, Chandravanshi BS, Leta S (2017) Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. ChemInt 3(4):291–305
Ranganathan K, Kabadgi SD (2011) Studies on feasibility of reverse osmosis (membrane) technology for treatment of tannery wastewater. J Environ Prot 2:37–46
De Aquim PM, Hansen É, Gutterres M (2019) Water reuse: an alternative to minimize the environmental impact on the leather industry. J Environ Manage 230:456–463
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Rights and permissions
About this article
Cite this article
Abdelkader, S.E., El-Gendy, A.S. & El-Haggar, S. Removal of trivalent chromium from tannery wastewater using solid wastes. Innov. Infrastruct. Solut. 6, 47 (2021). https://doi.org/10.1007/s41062-020-00414-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41062-020-00414-8