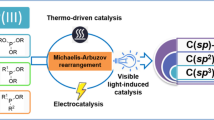
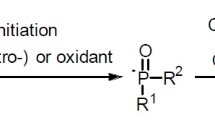
Abstract
As we all know, organic phosphorus compounds have high application values in chemical industries. Compared with traditional compounds with P–X (X = Cl, Br, I) and P–H bonds, phosphorylation reagents containing P(O)–OH bonds are stable, environmentally friendly, and inexpensive. However, in recent years, there have been few studies on the selective functionalization of P(O)–OH bonds for the fabrication of P–C and P–Z bonds. In general, four-coordinated P(O)–OH compounds have reached coordination saturation due to the phosphorus atom center, but cannot evolve the phosphorus coordination center through intra-molecular tautomerization; however, the weak coordination effects between the P=O bond and transition metals can be utilized to activate P(O)–OH bonds. This review highlights the most important recent contributions toward the selective functionalization of P(O)–OH bonds via cyclization/cross coupling/esterification reactions using transition metals or small organic molecules as the catalyst.



























































Similar content being viewed by others
References
Yang J, Chen T, Han LB (2015) J Am Chem Soc 137:1782–1785
Liu T, Li Y, Cheng F, Shen X, Liu J, Lin J (2019) Green Chem 21:3536–3541
Imamoto T, Saitoh Y, Koide A, Ogura T, Yoshida K (2007) Angew Chem Int Ed 46:8636–8639
Li KJ, Jiang YY, Xu K, Zeng CC, Sun BG (2019) Green Chem 21:4412–4421
Lu Y, Nakatsuji H, Okumura Y, Yao L, Ishihara K (2018) J Am Chem Soc 140:6039–6043
Nie SZ, Davison R, Dong V (2018) J Am Chem Soc 140:16450–16454
Unoh Y, Hirano K, Miura M (2017) J Am Chem Soc 139:6106–6109
Song S, Zhang Y, Yeerlan A, Zhu B, Liu J, Jiao N (2017) Angew Chem Int Ed 56:2487–2491
Guo H, Yoshimura A, Chen T, Saga Y, Han LB (2017) Green Chem 19:1502–1506
Quint V, Morlet-Savary F, Lohier JF, Lalevée J, Gaumont AC, Lakhdar S (2016) J Am Chem Soc 138:7436–7441
Gupta S, Baranwal S, Chaudharya P, Kandasamy J (2019) Org Chem Front 6:2260–2265
Chen T, Zhao CQ, Han LB (2018) J Am Chem Soc 140:3139–3155
Wang Y, Qian P, Su JH, Li Y, Bi M, Zha Z, Wang Z (2017) Green Chem 19:4769–4773
Chen XY, Pu M, Cheng HG, Sperger T, Schoenebeck F (2019) Angew Chem Int Ed 58:11395–11399
Zhu Y, Chen T, Li S, Shimada S, Han LB (2016) J Am Chem Soc 138:5825–5828
Peng AY, Ding YX (2003) J Am Chem Soc 125:15006–15007
Peng AY, Ding YX (2005) Org Lett 7:3299–3301
Sakakura A, Katsukawa M, Ishihara K (2007) Angew Chem Int Ed 46:1423–1426
Sakakura A, Sakuma M, Katsukawa M, Ishihara K (2008) Heterocycles 76:657–665
Coudray L, Bravo-Altamirano K, Montchamp JL (2008) Org Lett 10:1123–1126
Kanada J, Tanaka M (2011) Adv Synth Catal 353:890–896
Xu Q, Shen R, Ono Y, Nagahata R, Shimada S, Goto M, Han LB (2011) Chem Commun 47:2333–2335
Park Y, Seo J, Park S, Yoo EJ, Lee PH (2013) Chem Eur J 19:16461–16468
Park Y, Jeon I, Shin S, Min J, Lee PH (2013) J Org Chem 78:10209–10220
Ryu T, Kim J, Park Y, Kim S, Lee PH (2013) Org Lett 15:3986–3989
Unoh Y, Hashimoto Y, Takeda D, Hirano K, Satoh T, Miura M (2013) Org Lett 15:3258–3261
Eom D, Jeong Y, Kim YR, Lee E, Choi W, Lee PH (2013) Org Lett 15:5210–5213
Shin S, Jeong Y, Jeon WH, Lee PH (2014) Org Lett 16:2930–2933
Xiong B, Feng X, Zhu L, Chen T, Zhou Y, Au CT, Yin SF (2015) ACS Catal 5:537–543
Xiong B, Zeng K, Zhang S, Zhou Y, Au CT, Yin SF (2015) Tetrahedron 71:9293–9298
Xiong B, Cheng Q, Hu C, Zhang P, Liu Y, Tang K (2017) Chemistryselect 2:6891–6894
Qiao MM, Liu YY, Yao S, Ma TC, Tang ZL, Shi DQ, Xiao WJ (2019) J Org Chem 84:6798–6806
Trost BM, Spohr SM, Rolka AB, Kalnmals CA (2019) J Am Chem Soc 141:14098–14103
Wang G, Xiong B, Zhou C, Liu Y, Xu W, Yang CA, Tang KW, Wong WY (2019) Chem Asian J 14:4365–4374
Sakakura A, Katsukawa M, Ishihara K (2005) Org Lett 7:1999–2002
Yoshino T, Imori S, Togo H (2006) Tetrahedron 62:1309–1317
Sakakura A, Katsukawa M, Hayashi T, Ishihara K (2007) Green Chem 9:1166–1169
Wärme R, Juhlin L (2010) Phosphorus Sulfur Silicon 185:2402–2408
Kasemsuknimit A, Satyender A, Chavasiri W, Jang DO (2011) Bull Korean Chem Soc 32:3486–3488
Pu Y, Gao L, Liu H, Yan J (2012) Synthesis 44:99–103
Keglevich G, Kiss NZ, Mucsi Z, Körtvélyesi T (2012) Org Biomol Chem 10:2011–2018
Keglevich G, Kiss NZ, Drahos L, Körtvélyesi T (2013) Tetrahedron Lett 54:466–469
Jablonkai E, Milen M, Drahos L, Keglevich G (2013) Tetrahedron Lett 54:5873–5875
Jablonkai E, Henyecz R, Milen M, Koti J, Keglevich G (2014) Tetrahedron 70:8280–8285
Xiong B, Ye Q, Feng X, Zhu L, Chen T, Zhou Y, Au CT, Yin SF (2014) Tetrahedron 70:9057–9063
Qi N, Zhang N, Allu SR, Gao J, Guo J, He Y (2016) Org Lett 18:6204–6207
Zeng K, Chen L, Xiong B, Zhou Y, Au CT, Yin SF (2016) Tetrahedron Lett 57:2222–2226
Xiong B, Wang G, Zhou C, Liu Y, Li J, Zhang P, Tang K (2018) Phosphorus Sulfur Silicon 193:239–244
Xiong B, Hu C, Li H, Zhou C, Zhang P, Liu Y, Tang K (2017) Tetrahedron Lett 58:2482–2486
Xiong B, Wang G, Zhou C, Liu Y, Zhang P, Tang K (2018) J Org Chem 83:993–999
Li H, Lei J, Liu Y, Chen Y, Au CT, Yin SF (2019) Org Biomol Chem 17:302–308
Xiong B, Xu S, Zhu Y, Yao L, Zhou C, Liu Y, Tang KW, Wong WY (2020) Chem Eur J 26:9556–9560
Acknowledgements
This work was supported by National Natural Science Foundation of China (21606080), Natural Science Foundation of Hunan Province (2019JJ50203), Scientific Research Fund of Hunan Provincial Education Department (19A197), Innovation research group project of Natural Science Foundation of Hunan Province (no. 2020JJ1004) and Hunan Provincial Innovation Foundation for Postgraduate (CX20201132). W.-Y.W. thanks the Hong Kong Polytechnic University (1-ZE1C) and the Endowed Professorship in Energy from Ms Clarea Au (847S) for the financial support. P.-C. Q. thanks the Foundation of Wenzhou Science & Technology Bureau (no. W20170003) and the National Natural Science Foundation of China (no. 21828102) for support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiong, B., Xu, S., Liu, Y. et al. Recent Progress in the Selective Functionalization of P(O)–OH Bonds. Top Curr Chem (Z) 379, 5 (2021). https://doi.org/10.1007/s41061-020-00319-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-020-00319-1