Abstract
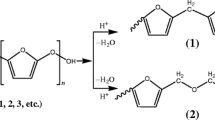
The degradation of poly(3-(oxiran-2-ylmethyl) oxazolidin-2-one) (poly(OMO)) has been studied. The reactions were carried out in the presence of an Algerian montmorillonite sheet silicate clay exchanged with protons called Maghnite-H+. First, the effect of solvent and reaction time on the conversion of monomer and the molecular weight are investigated in the polymerization of 3-(oxiran-2-ylmethyl) oxazolidin-2-one. Then, the rheological study of the obtained polymers in bulk and in solvent was conducted. Eventually, we notice that the obtained polymers degrade with time in the presence of Maghnite-H+.







Similar content being viewed by others
References
Akeb M, Harrane A, Belbachir M (2018) Polymerization of β-pinene by using natural montmorillonite clay as a green catalyst. Green Mater. https://doi.org/10.1680/jgrma.17.00040
Ayat M, Rahmouni A, Belbachir M (2016) Selective synthesis, characterization and kinetics studies of poly(α-Methyl styrene) induced by Maghnite-Na+ clay (Algerian MMT). Bull Chem React Eng Cat 11:376–388
Ayat M, Belbachir M, Rahmouni A (2017) Synthesis of block copolymers consists on vinylidene chloride and α-methylstyrene by cationic polymerization using an acid exchanged motmorillonite clay as heterogeneous catalyst (Algerian MMT). J Mol Struct 1139:381–389
Baghdadli MC, Meghabar R, Belbachir M (2016) Acid-activated algerian montmorillonite as heterogeneous catalysts for cationic polymerization of styrene. Asian J Chem 28(6):1197–1204
Ballantine JA (1992) Reactions assisted by clays and other lamellar solids – a survey (Ch. 4). In: Smith K (ed) Solid supports and catalysts in organic synthesis. Ellis Horwood Ltd., Chichester
Belbachir M, Bensaoula (2006) A composition and method for catalysis using bentonites. US Patent 7094823 B2
Bennabi S, Sahli N, Belbachir M, Brachais C-H, Boni G, Couvercelle J-P (2017) New approach for synthesis of poly(ethylglyoxylate) using Maghnite-H+, an Algerian proton exchanged montmorillonite clay. J Macromol Sci Part A Pur App Chem 54(1):1–10
Bentahar M, Meghabar R, Guemra K, Belbachir M (2017) A green catalyst for synthesis of bis-macromonomers of poly (styrene oxide). Rev Roum Chim 62(11):839–848
Breen C, Madejovà J, Komadel P (1995) High-pH alteration of argillaceous rocks: an experimental and modeling study. J Mater Chem 5(3):474–496
Brown DR (1994) Clays as catalyst and reagent support. Geol Carpath Ser Clay 45:45–56
Clarcks JH, Cullen SR, Barlow SJ, Bastock TW (1994) Environmentally friendly chemistry using supported reagent catalysts: structure–property relationships for clayzic. J Chem Soc Perkin Trans 2:1117–1130
Djamila CD, Meghabar R, Belbachir M (2017) Piperazine polymerization catalyzed by Maghnite-H+. J Environ Anal Chem 4(3):2380–2391
Hamam N, Ferrahi MI, Belbachir M (2016) Cationic ring opening copolymerization of 1,3-Dioxolane with styrene by montmorillonite Maghnite-H+ catalyst. Orient J Chem 32(3):1313–1317
Harrane A, Meghabar R, Belbachir M (2002) A protons exchanged montmorillonite clay as an efficient catalyst for the reaction of isobutylene polymerization. Int J Mol Sci 7:790–800
Hojabri F (1971) Gas-phase catalytic alkylation of aromatic hydrocarbons. J Appl Chem Biotechnol 21:87–89
Kaplan H (1966) General aniline and film corp. U.S.patent No 3,287,422
Medjdoub L, Rahmouni A, Belbachir M (2016) New method for nucleophilic substitution on hexachlorocyclotriphosphazene by allylamine using an Algerian proton exchanged montmorillonite clay (Maghnite-H+) as a green solid catalyst. Bull Chem React Eng Cat 11:151–160
Njopwouo D, Roques G, Wandji R (1987) A contribution to the study of the catalytic action of clays on the polymerisation of styrene: I characterization of polystyrenes. Clay Miner 22:145–156
Seghier S, Belbachir M (2016) Green polymerization of 4-(oxirane-2-ylmethyl) morpholine. Arab J Sci Eng 41:2171–2177
Solomon DH, Rosser MJ (1965) Reactions catalyzed by minerals. Part I. Polymerization of styrene. J Appl Polym Sci 9:1261–1271
Solomon DH, Swift JD (1967) Reactions catalyzed by minerals. Part II. Chain termination in free-radical polymerizations. J Appl Polym Sci 11:2557–2575
Thomas CL, Hickey J, Stecker G (1950) Chemistry of clay cracking catalyst. Ind Chem 42:866–871
Yahiaoui A, Belbachir M, Hachemaoui A (2003) An acid exchanged montmorillonite clay-catalyzed synthesis of polyepichlorohydrin. Int J Mol Sci 4:548–561
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seghier, S., Belbachir, M. Degradation of Poly(3-(Oxiran-2-ylmethyl) Oxazolidin-2-one) in the Presence of an Algerian Activated Clay. Iran J Sci Technol Trans Sci 43, 1545–1550 (2019). https://doi.org/10.1007/s40995-018-0629-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-018-0629-2