Abstract
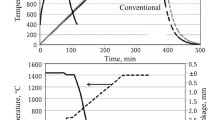
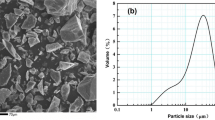
Nano-titania, up to 8 wt%, was added to magnesite–dolomite refractory matrix. The phase and microstructure analyses of samples heated up to 1650 °C for 3 h were studied by XRD and SEM/EDS, respectively. The physical properties are reported in terms of bulk density, apparent porosity, and hydration resistance. In addition, the mechanical behavior was studied by a cold crushing strength (CCS), and flexural strength at 1200 °C test. As a result, it was found that the presence of nano-TiO2 in the magnesite–dolomite matrix induced titanates formation (Mg2TiO4 and CaTiO3), which improved the sintering process. Nano-titania influenced the bonding structure through a direct bonding enhancement. In general, the addition of 6 wt% of nano-TiO2 contributed to reach a maximum increment in physical and mechanical properties.







Similar content being viewed by others
References
Adak S, Bal AS, Chattopadhyay AK, Panda PB, Rana RP (2011) Effect of nano-titania addition on the properties of magnesia–carbon system. In: Proceedings of the 54th International Colloquium on Refractories, Aachen, Germany, pp 180–183
Antonovic V, Pundiene I, Stonys R, Cesniene J, Keriene J (2010) A review of the possible applications of nanotechnology in refractory concrete. J Civil Eng Manag 16(4):595–602
Azhari A, Golestani-Fard F, Sarpoolaky H (2009) Effect of nano iron oxide as an additive on phase and microstructural evolution of mag-chrome refractory matrix. J Eur Ceram Soc 29(13):2679–2684
Chaudhuri M, Banerjee G, Kumar A, Sarkar SL (1999) Secondary phases in natural magnesite sintered with addition of titania, ilmenite and zirconia. J Mater Sci 34:5821–5825
Chen S, Chen G, Cheng J (2000) Effect of additive on the hydration resistance of material synthesized from the magnesia-calcia system. J Am Ceram Soc Process 83:1810–1812
Chen M, Jin A, Wang N, Yu J (2006) Synthesis of hydration–resistance of CaO refractory by addition of MgO. J Min Process 14:409–416
Chen M, Wang N, Jingkun Y, Yamaguchi A (2007a) Effect of porosity on carbonation and hydration of CaO material. J Eur Ceram Soc 27:1953–1959
Chen M, Lu C, Yu J (2007b) Improvement in performance of MgO–CaO refractories by addition of nano-sized ZrO2. J Eur Ceram Soc 27:4633–4638
Ghasemi-Kahrizsangi S, Nemati A, Shahraki A, Farooghi M (2016a) Densification and Properties of Fe2O3 Nanoparticles added CaO Refractories. Ceram Int 42:12270–12275
Ghasemi-Kahrizsangi S, Nemati A, Shahraki A, Farooghi M (2016b) Effect of nano-sized Fe2 O3 on microstructure and hydration resistance of MgO–CaO Refractories. Int J Nanosci Nanotechnol 12:19–26
Ghasemi-Kahrizsangi S, Barati M, Gheisari H, Shahraki A, Farooghi M (2016c) Densification and properties of ZrO2 nanoparticles added magnesia—doloma refractories. Ceram Int 29:15658–15663
Ghosh A, Tripathi HS (2012) Sintering behavior and hydration resistance of reactive dolomite. J Ceram Int 38:1315–1318
Ghosh A, Bhattacharay TK, Mukherjee B, Das SK (2004) Densification and properties of lime with V2O5 additions. J Ceram Int 30:2117–2120
Ghosh A, Bhattacharay TK, Mukherjee B, Das SK (2011) The effect of CuO addition on the sintering of lime. J Ceram Int 27:201–203
Golubović A, Radović M (2011) The growth of Mg2TiO4 single crystals using a four-mirror furnace. J Serbian Chem Soc 76(11):1561–1566
Gudilina AI, Pitak NV, Kushchenko AV (1984) Certain properties of MgO–Cr2O3–TiO2 compositions. Refract Ind Ceram 25(9):559–562
Guo R, Jiang Y, Bhalla AS (1998) Growth and properties of CaTiO3 single Crystalfibers. J Electroceram 2(3):199–203
Han B, Li Y, Guo C, Li N, Chen F (2007) Sintering of MgO-based refractories with added WO3. Ceram Int 33(8):1563–1567
Huizhong LH, Jianxiu W (2013) Influence of nano-Fe2O3 on sintering and mechanical property of magnesia-chrome refractories. Refractories 5:002
Kashaninia F, Arpoolaky HS, Naghizadeh R, Bagheri AR, Zamanipour M (2011) Improving hydration resistance of magnesia-doloma refractories by iron oxide addition. Iran J Mater Sci Eng 8:34-40
Khlebnikova Y, Zhukovskaya AE, Seliovanova AN (2007) Methods for determining hydration resistance of refractories. J Refract Ind Ceram 48:2–6
Khoroshavin LB, Perepelitsyn VA (1999) On the nanotechnology of refractories. Refract Ind Ceram 40:553–557
Kuznetsov DV, Lysov DV, Nemtinov AA, Shaleiko AS, Korolkov VA (2010) Nanomaterials in refractory technology. Refract Ind Ceram 5:61–63
Lee YB, Park HC, Oh KD (1998) Sintering and microstructure development in the system MgO–TiO2. J Mater Sci 33:4321–4325
Lucion T, Duvigneaud PH, Laudet A, Stenger JF, Gueguen E (2004) Effect of TiO2 additions on the densification of MgO and MgO–CaO mixtures. Key Eng Mater 264–268:209–212
Manivasakan P, Rajendran V, Rauta PR, Sahu BB, Sahu P, Panda BK, Valiyaveettill S, Jegadesan S (2010) Effect of TiO2 nanoparticles on properties of silica refractory. J Am Ceram Soc 93(8):2236–2243
Martinac V, Labor M, Petric N (1996) Effect of TiO2, SiO2 and Al2O3on properties of sintered magnesium oxide from sea water. Mater Chem Phys 46:23–30
Otham AGM, Abuel MA, Serry MA (2001) Hydration–resistant lime refractories from egyption lime stone and ilmenite raw materials. Ceram Int 27:801–807
Othman AGM (2003) Effect of talc and bauxite on sintering, microstructure and refractory properties of Egyptian dolomitic magnesite. Br Ceram Trans 102(6):265–271
Petrović V (2006) Sintering kinetics of MgO–TiO2systems. Sci Sinter 38:287–292
Pivinskii YuE, Dyakin PV, Ya Yu, Pivinskii SV Vikhman (2003) Nanoparticles and their effective use in the technology of highly concentrated binding suspensions (hcbs) and refractory castables. Refract Ind Ceram 44:314–318
Rodríguez E, Moreno FH, Aguilar-Martínez JA, Montes-Mejía AE, Ruiz-Valdés JJ, Puente-Ornelas R, Contreras JE (2016) effect of nano-titania (n-Tio2) content on the mechano-physical properties of a magnesia refractory composite. Ceram Int 42:8445–8452
Suvorov SA, Nazmiev MI, Polovinkina RS, Maryasev IG (2006) Water-resists lime-magnesia clinker. J Refract Ind Ceram 47:38–40
Tamura S, Ochiai T, Takanaga S, Kanai T, Nakamura H (2003) Nano-tech refractories 1: the development of the nanostructural matrix. In: Proceedings of UNITECR’03 Congress, 19–22 October, Osaka, Japan, pp 517–520
Yin H, Ma Y, Yan J (2011) Effect of MgO coating on hydration resistance of MgO-CaO clinkers. J Mater Sci Forum 695:324–327
Zargar HR, Oprea C, Oprea G, Troczynski T (2012) The effect of nano-Cr2O3 on solid-solution assisted sintering of MgO refractories. Ceram Int 38(8):6235–6241
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi-Kahrizsangi, S., Shahraki, A. & Farooghi, M. Effect of Nano-TiO2 Additions on the Densification and Properties of Magnesite–Dolomite Ceramic Composites. Iran J Sci Technol Trans Sci 42, 567–575 (2018). https://doi.org/10.1007/s40995-016-0143-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-016-0143-3