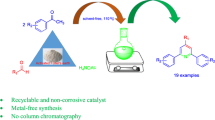
Abstract
Henry reaction is a base-catalyzed reaction which can produce β-nitroalcohol or nitroethylene derivatives from the condensation of aryl aldehydes and nitromethane under different conditions. In the present article, we introduce hexamethylenetetramine as a basic organocatalyst for Henry reaction, which can produce β-nitroalcohol derivatives from the condensation of aryl aldehydes and nitromethane under different conditions. By application of this organic base, β-nitroalcohol compounds were produced as the only product in good to excellent yields. In addition, reusability of hexamethylenetetramine in this reaction makes our method green.

Similar content being viewed by others
References
Ballini R, Bosica G, Livi D, Palmieri A, Maggi R, Sartori G (2003) Use of heterogeneous catalyst KG-60-NEt2 in Michael and Henry reactions involving nitroalkanes. Tetrahedron Lett 44:2271–2273
Boruwa J, Barua NC (2006) Stereoselective total synthesis of (+)-boronolide. Tetrahedron 62:1193–1196
Caldarelli M, Habermann J, Ley SV (1999) Clean five step synthesis of an array of 1,2,3,4-tetra-subsitutated pyrroles using polymer supported reagents. J Org Chem Perkin Trans 1:107–110
Cao C, Lu Z, Cai Z et al (2012) Cheap Cu(I)/hexamethylenetetramine (HMTA) catalytic system for C–N coupling reactions. Synth Commun 42:279–284
Cwik A, Fuchs A, Hell Z, Clacens JM (2005) Nitroaldol-reaction of aldehydes in the presence of non-activated Mg: Al 2:1 hydrotalcite; a possible new mechanism for the formation of 2-aryl-1,3-dinitropropanes. Tetrahedron 61:4015–4021
Dalko PI, Moisan L (2004) In the golden age of organocatalysis. Angew Chem Int Ed 43:5138–5175
Fatehi M, Saleh TM, Fatehi-Hassanabad Z, Farrokhfal K, Jafarzadeh M, Davodi S (2005) A pharmacological study on Berberis vulgaris fruit extract. J Ethnopharmacol 102:46–52
Gan C, Chen X, Lai G, Wang Z (2006) Rapid microwave-assisted Henry reaction in solvent-free processes. Synlett 387–390
Han J, Xu Y, Su Y, She X, Pan X (2008) Guanidine-catalyzed Henry reaction and Knoevenagel condensation. Catal Commun 9:2077–2079
Hara T, Kanai S, Mori K, Mizugaki T, Ebitani K, Jitsukawa K, Kaneda K (2006) Highly efficient C–C bond-forming reactions in aqueous media catalyzed by monomeric vanadate species in an apatite framework. J Org Chem 71:7455–7462
Henry LCR (1895) Highly enantioselective Henry (nitroaldol) reaction of aldehydes and α-ketoesters catalyzed by N, N′-dioxide-copper(I) complexes. Hebd Seances Acad Sci 120:1265–1268
Hirata N, Hayashi M (2007) Nitroaldol reaction catalyzed by tris(2,4,6-trimethoxyphenyl) phosphine (TTMPP). Synth Commun 37:1653–1657
Kaur N, Kishore D (2013) An insight into hexamethylenetetramine: a versatile reagent in organic synthesis. J Iran Chem Soc 10:1193–1228
Khosravi K, Kazemi S (2012) Synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles in both room temperature and microwave condition catalyzed by hexamethylenetetramine–bromine complex. Chin Chem Lett 23:61–64
Li Y, Feng JP, Wang WH, Chen J, Cao XP (2007) Total synthesis and correct absolute configuration of malyngamide U. J Org Chem 72:2344–2350
Luzzio FA (2001) The Henry reaction: recent examples. Tetrahedron 57:915–1138
Majhi A, Kadam ST, Kim SS (2009) TMEDA catalyzed Henry (nitroaldol) reaction under metal and solvent-free conditions. Bull Korean Chem Soc 30:1767–1770
McNulty J, Dyck V, Larichev A, Capretta A, Robertson J (2004) Phosphonium salt catalyzed Henry nitroaldol reactions. Lett Org Chem 1:137–139
Palacios F, De los Santos JM, Aparicio D (2005) Use of polymer-supported amines in the catalytic nitroaldol reaction of nitroalkanes with aldehydes. Arkivok ix:405–414
Phukan M, Borah KJ, Borah R (2008) Imidazole catalyzed Henry reactions in aqueous medium. Synth Commun 38:3068–3073
Rosini G (1991) The Henry (nitroaldol) reaction. In: Trost BM, Fleming I (eds) Comprehensive organic synthesis. Pergamon, New York, pp 321–340
Sato KI, Akai S, Shoji H, Sugita N, Yoshida S, Nagai Y, Suzuki K, Nakamura Y, Kajihara Y, Funabashi M, Yoshimura J (2008) Stereoselective and efficient total synthesis of optically active tetrodotoxin from d-glucose. J Org Chem 73:1234–1242
Shinde PS, Shinde SS, Dake SA, Sonekar VS, Sonekar VS, Deshmukh SU, Thorat VV, Andurkar NM, Pawar RP (2010) CsF/[bmim][BF4]: an efficient and reusable system for Henry. Arab J Chem. doi:10.1016/j.arabjc.2010.12.028.reaction
Simoni D, Rondanin R, Morini M, Baruchello R, Invidiata FP (2000) 1,5,7-Triazabicyclo[4.4.0]dec-1-ene (TBD), 7-methyl-TBD (MTBD) and the polymer-supported TBD (P-TBD): three efficient catalysts for the nitroaldol (Henry) reaction and for the addition of dialkyl phosphites to unsaturated systems. Tetrahedron Lett 41:1607–1610
Vovard-Le Bray C, Jiang F, Wu XF, Sortais JB, Darcel C (2010) Cyclen-catalyzed Henry reaction under neutral conditions. Tetrahedron Lett 51:4555–4557
Wang X, Fang F, Zhao C, Tian SK (2008) Dual-reagent organocatalysis with a phosphine and electron-deficient alkene: application to the Henry reaction. Tetrahedron Lett 49:6442–6448
Weeden JA, Chisholm JD (2006) Phosphine-catalyzed nitroaldol reactions. Tetrahedron Lett 47:9313–9578
Yadav JS, Reddy BVS, Basak AK, Visali B, Narsaiah AV, Nagaiah K (2004) Phosphane-catalyzed knoevenagel condensation: a facile synthesis of α-cyanoacrylates and α-cyanoacrylonitrile. Eur J Org Chem 3:546–551
Youn SW, Kim YH (2000) Facile synthesis of 2-nitroalkanols mediated with LiAlH4 as catalyst. Synlett 880–882
Zhou GP, Hui YH, Wan NN, Liu QJ, Xie ZF, Wang JD (2012) Mn(OAc)2/Schiff base as a new efficient catalyst system for the Henry reaction of nitroalkanes with aldehydes. Chin Chem Lett 23:690–694
Acknowledgments
We are grateful to the Research Council of Shiraz University for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fathi, S., Sardarian, A.R. Hexamethylenetetramine as an Efficient and Reusable Organocatalyst in Henry Reaction Under Mild and Aqueous Solution. Iran. J. Sci. Technol. Trans. Sci. 40, 103–107 (2016). https://doi.org/10.1007/s40995-016-0002-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-016-0002-2