Abstract
Purpose
The knee is a complex joint that can be harmed by injuries including osteochondral defects and degenerative diseases such as osteoarthritis (OA). In this context, one of the most promising treatments is the use of hydrogels enriched with mesenchymal stem cells (MSC). Therefore, the aim of this study was to perform a systematic literature review to identify the main biological changes caused by MSC-enriched hydrogels for the treatment of osteochondral defects and osteoarthritic knees using in vivo experimental models.
Methods
This review was carried out between January and June 2023 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), and the SYRCLE risk of bias tool was used to assess study quality.
Results
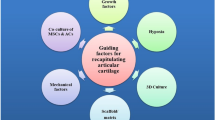
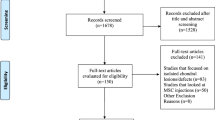
A total of 1254 articles were retrieved from the Embase, Cochrane, Web of Science, PubMed, and Lilacs databases. After sorting the ar7ticles, 11 were included in this work. It is possible to observe that there is heterogeneity of materials used for the hydrogel manufacturing and the use of MSCs from different origins. It is also possible to observe that the groups treated with MSC-enriched hydrogel had smooth cartilaginous tissue surfaces and a higher amount of glycosaminoglycan (GAG) deposition. Also, a higher immunostaining of collagen II, GAG, and aggrecan and lower levels of collagen X and pro-inflammatory cytokines were observed.
Conclusion
In conclusion, this review demonstrates that MSC-enriched hydrogels presented very positive effects on the process of cartilage healing.
Lay Summary
A systematic literature review was conducted to identify the biological changes caused by mesenchymal stem cell (MSC)-enriched hydrogels in the treatment of osteochondral defects and osteoarthritic knees. Out of 1254 retrieved articles, 11 were included in the review. The study found a variety of materials used for hydrogel manufacturing and MSCs from different sources. The groups treated with MSC-enriched hydrogel showed smoother cartilaginous tissue surfaces; increased glycosaminoglycan (GAG) deposition; higher immunostaining of collagen II, GAG, and aggrecan; and lower levels of collagen X and pro-inflammatory cytokines. Overall, the review concludes that MSC-enriched hydrogels have positive effects on cartilage healing.


Similar content being viewed by others
Data Availability
The data supporting the results of this study will be made available by the corresponding author upon reasonable request.
References
Kaderli S, Boulocher C, Pillet E, Watrelot-Virieux D, Rougemont AL, Roger T, et al. A novel biocompatible hyaluronic acid-chitosan hybrid hydrogel for osteoarthrosis therapy. Int J Pharm [Internet]. 2015 [cited 2023 Mar 30];483(1–2):158–68. Available from: https://pubmed.ncbi.nlm.nih.gov/25666331/
Wyszyńska J, Bal-Bocheńska M. Efficacy of high-intensity laser therapy in treating knee osteoarthritis: a first systematic review. Photomed Laser Surg [Internet]. 2018 [cited 2023 Mar 30]; 36(7):343–53. Available from: https://pubmed.ncbi.nlm.nih.gov/29688827/
Badekas T, Takvorian M, Souras N. Treatment principles for osteochondral lesions in foot and ankle. Int Orthop [Internet]. 2013 [cited 2023 Mar 30];37(9):1697. Available from: /pmc/articles/PMC3764304/
Grover A, Sanjuan-Pla A, Thongjuea S, Carrelha J, Giustacchini A, Gambardella A, et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nature Communications [Internet]. 2016 [cited 2023 Apr 19];7(1):1–12. Available from: https://www.nature.com/articles/ncomms11075
Huang T Le, Hsu HC, Yang KC, Yao CH, Lin FH. Effect of different molecular weight hyaluronans on osteoarthritis-related protein production in fibroblast-like synoviocytes from patients with tibia plateau fracture. J Trauma [Internet]. 2010 [cited 2023 Mar 30];68(1):146–52. Available from: https://pubmed.ncbi.nlm.nih.gov/20065770/
Ha CW, Park YB, Chung JY, Park YG. Cartilage repair using composites of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel in a minipig model. Stem Cells Transl Med [Internet]. 2015 [cited 2023 Mar 30];4(9):1044–51. Available from: https://pubmed.ncbi.nlm.nih.gov/26240434/
Yang J, Zhang YS, Yue K, Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25.
Benmassaoud MM, Gultian KA, DiCerbo M, Vega SL. Hydrogel screening approaches for bone and cartilage tissue regeneration. Ann N Y Acad Sci [Internet]. 2020 [cited 2023 Mar 30];1460(1):25–42. Available from: https://www.researchwithrowan.com/en/publications/hydrogel-screening-approaches-for-bone-and-cartilage-tissue-regen
Ohmes J, Saure LM, Schütt F, Trenkel M, Seekamp A, Scherließ R, et al. Injectable thermosensitive chitosan-collagen hydrogel as a delivery system for marine polysaccharide fucoidan. Mar Drugs [Internet]. 2022 [cited 2023 Mar 30];20(6). Available from: https://pubmed.ncbi.nlm.nih.gov/35736205/
Choi JH, Choi OK, Lee J, Noh J, Lee S, Park A, et al. Evaluation of double network hydrogel of poloxamer-heparin/gellan gum for bone marrow stem cells delivery carrier. Colloids Surf B Biointerfaces [Internet]. 2019 [cited 2023 Mar 30];181:879–89. Available from: https://pubmed.ncbi.nlm.nih.gov/31382336/
Wang AT, Zhang QF, Wang NX, Yu CY, Liu RM, Luo Y, et al. Cocktail of hyaluronic acid and human amniotic mesenchymal cells effectively repairs cartilage injuries in sodium iodoacetate-induced osteoarthritis rats. Front Bioeng Biotechnol. 2020;6:8.
Yan X, Yang B, Chen Y, Song Y, Ye J, Pan Y, et al. Anti-friction MSCs delivery system improves the therapy for severe osteoarthritis. Adv Mater. 2021;33(52).
Thakur A, Jaiswal MK, Peak CW, Carrow JK, Gentry J, Dolatshahi-Pirouz A, et al. Injectable shear-thinning nanoengineered hydrogels for stem cell delivery. Nanoscale. 2016;8(24):12362–72. https://doi.org/10.1039/c6nr02299e.
Bhattacharjee M, Escobar Ivirico JL, Kan HM, Shah S, Otsuka T, Bordett R, et al. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc Natl Acad Sci USA [Internet]. 2022;119(4):e2120968119. https://doi.org/10.1073/pnas.2120968119.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg [Internet]. 2010 [cited 2023 Apr 4];8(5):336–41. Available from: https://pubmed.ncbi.nlm.nih.gov/20171303/
Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol [Internet]. 2014 [cited 2023 Apr 19];14(1). Available from: https://pubmed.ncbi.nlm.nih.gov/24667063/
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol [Internet]. 2011 [cited 2023 Apr 4];64(4):401–6. Available from: https://pubmed.ncbi.nlm.nih.gov/21208779/
Qi BW, Yu AX, Zhu SB, Zhou M, Wu G. Chitosan/poly(vinyl alcohol) hydrogel combined with ad-htgf-b1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med. 2013;238(1):23–30.
Kim JK, Bae HC, Ro DH, Lee S, Lee MC, Han HS. Enhancement of cartilage regeneration of synovial stem cells/hydrogel by using transglutaminase-4. Tissue Eng Part A. 2021;27(11–12):761–70.
Liu H, Rui Y, Liu J, Gao F, Jin Y. Hyaluronic acid hydrogel encapsulated BMP-14-modified ADSCs accelerate cartilage defect repair in rabbits. J Orthop Surg Res. 2021;16(1).
Vayas R, Reyes R, Arnau MR, Évora C, Delgado A. Injectable scaffold for bone marrow stem cells and bone morphogenetic protein-2 to repair cartilage. Cartilage [Internet]. 2021 [cited 2023 Apr 4];12(3):293. Available from: /pmc/articles/PMC8236655/
Wang AT, Zhao M, Feng Y, Jia H, Zhang L, Yu H, et al. Multifaceted optimization of MSC-based formulation upon sodium iodoacetate-induced osteoarthritis models by combining advantageous HA/PG hydrogel and fluorescent tracer. Stem Cells Int. 2021; 2021.
Wu KC, Chang YH, Liu HW, Ding DC. Transplanting human umbilical cord mesenchymal stem cells and hyaluronate hydrogel repairs cartilage of osteoarthritis in the minipig model. Tzu Chi Med J. 2019;31(1):11–9.
Ha CW, Park YB, Chung JY, Park YG. Cartilage repair using composites of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel in a minipig model. Stem Cells Transl Med. 2015;4(9):1044–51.
Bhattacharjee M, Escobar Ivirico JL, Kan HM, Shah S, Otsuka T, Bordett R, et al. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc Natl Acad Sci USA. 2022;119(4).
Liu C, Liu D, Wang Y, Li Y, Li T, Zhou Z, et al. Glycol chitosan/oxidized hyaluronic acid hydrogels functionalized with cartilage extracellular matrix particles and incorporating BMSCs for cartilage repair. Artif Cells Nanomed Biotechnol. 2018;46(sup1):721–32.
Jia Z, Zhu F, Li X, Liang Q, Zhuo Z, Huang J, et al. Repair of osteochondral defects using injectable chitosan-based hydrogel encapsulated synovial fluid-derived mesenchymal stem cells in a rabbit model. Mater Sci Eng C. 2019;1(99):541–51.
Meng X, Ziadlou R, Grad S, Alini M, Wen C, Lai Y, et al. Animal models of osteochondral defect for testing biomaterials. Biochem Res Int [Internet]. 2020 [cited 2023 Apr 19];2020. Available from: https://pubmed.ncbi.nlm.nih.gov/32082625/
Chu, C. R., Szczodry, M., & Bruno, S. (2010). Animal models for cartilage regeneration and repair. Tissue Engineering, Part B, Reviews, 16(1), 105–115. https://doi.org/10.1089/ten.TEB.2009.0452
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy [Internet]. 2006 [cited 2023 Apr 19];8(4):315–7. Available from: https://pubmed.ncbi.nlm.nih.gov/16923606/
Hashemi M, Kalalinia F. Application of encapsulation technology in stem cell therapy. Life Sci. 2015;15(143):139–46.
Gasperini L, Mano JF, Reis RL. Natural polymers for the microencapsulation of cells. J R Soc Interface [Internet]. 2014; 11(100). https://doi.org/10.1098/rsif.2014.0817
Ma Q, Liao J, Cai X. Different sources of stem cells and their application in cartilage tissue engineering. Curr Stem Cell Res Ther [Internet]. 2018 [cited 2023 Apr 19];13(7):568–75. Available from: https://pubmed.ncbi.nlm.nih.gov/29359678/
Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev [Internet]. 2014 [cited 2023 Apr 19];20(6):596–608. Available from: https://pubmed.ncbi.nlm.nih.gov/24749845/
Wan W, Li Q, Gao H, Ge L, Liu Y, Zhong W, et al. BMSCs laden injectable amino-diethoxypropane modified alginate-chitosan hydrogel for hyaline cartilage reconstruction. J Mater Chem B. 2015;3(9):1990–2005.
Han L, Liu K, Wang M, Wang K, Fang L, Chen H, et al. Mussel-inspired adhesive and conductive hydrogel with long-lasting moisture and extreme temperature tolerance. Adv Funct Mater. 2018;28(3):1–12.
Oprenyeszk F, Chausson M, Maquet V, Dubuc JE, Henrotin Y. Protective effect of a new biomaterial against the development of experimental osteoarthritis lesions in rabbit: a pilot study evaluating the intra-articular injection of alginate-chitosan beads dispersed in an hydrogel. Osteoarthr Cartil. 2013;21(8):1099–107.
Echalier C, Valot L, Martinez J, Mehdi A, Subra G. Chemical cross-linking methods for cell encapsulation in hydrogels. Mater Today Commun. 2019;1:20.
Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584–94. https://doi.org/10.1038/nrrheum.2013.109.
Manferdini C, Maumus M, Gabusi E, Piacentini A, Filardo G, Peyrafitte J, Jorgensen C, Bourin P, Fleury-Cappellesso S, Facchini A, Noël D, Lisignoli G. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E 2. Arthritis Rheum. 2013;65(5):1271–81. https://doi.org/10.1002/art.37908.
Van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JAN, Bernsen MR, van Osch GJVM. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr Carti. 2012;20(10):1186–96. https://doi.org/10.1016/j.joca.2012.06.003.
Funding
This study was financed by the São Paulo Research Foundation (FAPESP), grants #2019/10228–5 and #2022/13515–8.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and data analysis were performed by MB, LCSS, and HGM. The analyses of the levels of evidence and methodological quality of the included studies were performed by JRP and ALMA. Authors CCSM, LA, and ACMR were responsible for study writing and review. Also, DAR assisted in the review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The research was not submitted to an ethics committee, as it was a review study. This systematic review has been registered on PROSPERO with CRD4202339451.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bonifacio, M., Garcia-Motta, H., Martignago, C.C.S. et al. Mesenchymal Stem Cell-Enriched Hydrogels for the Treatment of Knee Joint Disorders: A Systematic Review. Regen. Eng. Transl. Med. (2024). https://doi.org/10.1007/s40883-024-00339-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40883-024-00339-z