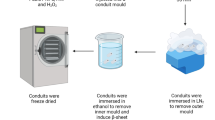
Abstract
Purpose
There are more than 600 conditions that can lead to nerve tissue loss and there is hardly any treatment other than the good old autologous nerve graft transplant to surpass the negative outcomes that result. Thus, nerve guide scaffolds are being essentially developed to fruitfully put stem cell-based therapy to use for nerve tissue repair and neuroregeneration.
Methods
We performed a literature search for studies on variety of electroconductive and electroactive scaffolds and conduits from different materials such as polypyrrole, polyurethane, graphene, carbon nanotubes, polycaprolactone and silk have been developed to achieve the aim of neuroregeneration.
Results
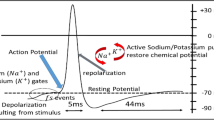
The essential role of electrical stimulation (ES) and signaling has been realised in helping differentiation, proliferation, myelination and migration of neuronal cells along with axonal and neurite outgrowth. ES has also shown improved neurotrophic secretion by Schwann cells thereby increasing the chances of an efficient and functional regenerated nerve.
Conclusion
Electroconductive and electroactive nerve guide scaffolds and conduits are being essentially developed to fruitfully put stem cell-based therapy to use for nerve tissue repair and neuroregeneration.
Lay Summary
In the development of electroconductive and electroactive nerve guide scaffolds and conduits, it is important to recreate all the characteristics of functional nerve tissue in their natural and functional form with appropriate mechanical strength and biocompatibility.
Hence, natural or synthetic biomaterials incorporated with electroconductive and electroactive potential may lead to a new generation of nerve conduits.
The following review sufficiently provides a detailed insight into the current research and future implications.


Similar content being viewed by others
References
Ababneh NA, Al-Kurdi B, Jamali F, Awidi A. A comparative study of the capability of MSCs isolated from different human tissue sources to differentiate into neuronal stem cells and dopaminergic-like cells. PeerJ. 2022;10:e13003. https://doi.org/10.7717/peerj.13003.
Alexander JK, Fuss B, Colello RJ. Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol. 2006;2(2):93–103. https://doi.org/10.1017/S1740925X0600010X.
An B, Ma Y, Xu Y, Liu X, Zhang X, Zhang J, Yang C. Crocin regulates the proliferation and migration of neural stem cells after cerebral ischemia by activating the Notch1 pathway. Folia Neuropathol. 2020;58(3):201–12. https://doi.org/10.5114/fn.2020.100063.
Anderson M, Shelke NB, Manoukian OS, Yu X, McCullough LD, Kumbar SG. Peripheral nerve regeneration strategies: electrically stimulating polymer based nerve growth conduits. Crit Rev Biomed Eng. 2015;43(2–3):131–59. https://doi.org/10.1615/CritRevBiomedEng.2015014015.
BahremandiTolou N, Salimijazi H, Kharaziha M, Faggio G, Chierchia R, Lisi N. A three-dimensional nerve guide conduit based on graphene foam/polycaprolactone. Mater Sci Eng C Mater Biol Appl. 2021;126:112110. https://doi.org/10.1016/j.msec.2021.112110.
Baniasadi H, Ramazani S A A, Mashayekhan S. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int J Biol Macromol. 2015;74:360–6. https://doi.org/10.1016/j.ijbiomac.2014.12.014.
Barroca N, Marote A, Vieira SI, Almeida A, Fernandes MHV, Vilarinho PM, da Cruz E, Silva OAB. Electrically polarized PLLA nanofibers as neural tissue engineering scaffolds with improved neuritogenesis. Colloids Surf B Biointerfaces. 2018;167:93–103. https://doi.org/10.1016/j.colsurfb.2018.03.050.
Bertleff MJOE, Meek MF, Nicolai J-PA. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg. 2005;30(3):513–8. https://doi.org/10.1016/j.jhsa.2004.12.009.
Blozovski D. Potentials evoked by the electric stimulation of the optic nerve in chick embryo. J Physiol. 1969;61(Suppl 2):225.
Boroujeni ME, Gardaneh M. Umbilical cord: an unlimited source of cells differentiable towards dopaminergic neurons. Neural Regen Res. 2017;12(7):1186–92. https://doi.org/10.4103/1673-5374.211201.
Burks SS, Diaz A, Haggerty AE, de la Oliva N, Midha R, Levi AD. Schwann cell delivery via a novel 3D collagen matrix conduit improves outcomes in critical length nerve gap repairs. J Neurosurg. 2021;135(4):1241–51. https://doi.org/10.3171/2020.8.JNS202349.
Catania F, Marras E, Giorcelli M, Jagdale P, Lavagna L, Tagliaferro A, Bartoli M. A review on recent advancements of graphene and graphene-related materials in biological applications. Appl Sci. 2021;11(2):2. https://doi.org/10.3390/app11020614.
Chato-Astrain J, Campos F, Roda O, Miralles E, Durand-Herrera D, Sáez-Moreno JA, García-García S, Alaminos M, Campos A, Carriel V. In vivo evaluation of nanostructured fibrin-agarose hydrogels with mesenchymal stem cells for peripheral nerve repair. Front Cell Neurosci. 2018;12:501. https://doi.org/10.3389/fncel.2018.00501.
Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. https://doi.org/10.1186/s12967-014-0253-7.
Cheng L-N, Duan X-H, Zhong X-M, Guo R-M, Zhang F, Zhou C-P, Shen J. Transplanted neural stem cells promote nerve regeneration in acute peripheral nerve traction injury: assessment using MRI. AJR Am J Roentgenol. 2011;196(6):1381–7. https://doi.org/10.2214/AJR.10.5495.
Choi SJ, Park SY, Shin YH, Heo S-H, Kim K-H, Lee HI, Kim JK. Mesenchymal stem cells derived from Wharton’s jelly can differentiate into Schwann cell-like cells and promote peripheral nerve regeneration in acellular nerve grafts. Tissue Eng Regen Med. 2021;18(3):467–78. https://doi.org/10.1007/s13770-020-00329-6.
Conductive-polymers | Sigma-Aldrich (n.d.) Retrieved November 28, 2023, from https://www.sigmaaldrich.com/IN/en/search/conductive-polymers?focus=products&page=1&perpage=30&sort=relevance&term=conductive-polymers&type=product.
di Summa PG, Kingham PJ, Campisi CC, Raffoul W, Kalbermatten DF. Collagen (NeuraGen®) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci Lett. 2014;572:26–31. https://doi.org/10.1016/j.neulet.2014.04.029.
Do JL, Allahwerdy S, David RCC, Weinreb RN, Tuszynski MH, Welsbie DS. Optic nerve engraftment of neural stem cells. Invest Ophthalmol Vis Sci. 2021;62(9):30. https://doi.org/10.1167/iovs.62.9.30.
Dobrzański LA, Hudecki A, Chladek G, Król W, Mertas A. Biodegradable and antimicrobial polycaprolactone nanofibers with and without silver precipitates. Arch Mater Sci Eng. 2015;76(1):5–26.
Donoghoe N, Rosson GD, Dellon AL. Reconstruction of the human median nerve in the forearm with the Neurotube. Microsurgery. 2007;27(7):595–600. https://doi.org/10.1002/micr.20408.
Du L, Li T, Jin F, Wang Y, Li R, Zheng J, Wang T, Feng Z-Q. Design of high conductive and piezoelectric poly (3,4-ethylenedioxythiophene)/chitosan nanofibers for enhancing cellular electrical stimulation. J Colloid Interface Sci. 2020;559:65–75. https://doi.org/10.1016/j.jcis.2019.10.003.
Egeland BM, Urbanchek MG, Peramo A, Richardson-Burns SM, Martin DC, Kipke DR, Kuzon WM, Cederna PS. In vivo electrical conductivity across critical nerve gaps using poly(3,4-ethylenedioxythiophene)-coated neural interfaces. Plast Reconstr Surg. 2010;126(6):1865–73. https://doi.org/10.1097/PRS.0b013e3181f61848.
Escobar A, Serafin A, Carvalho MR, Culebras M, Cantarero A, Beaucamp A, Reis RL, Oliveira JM, Collins MN. Electroconductive poly(3,4-ethylenedioxythiophene) (PEDOT) nanoparticle-loaded silk fibroin biocomposite conduits for peripheral nerve regeneration. Adv Compos Hybrid Mater. 2023;6(3):118. https://doi.org/10.1007/s42114-023-00689-2.
Fabbro A, Cellot G, Prato M, Ballerini L. Interfacing neurons with carbon nanotubes: (re)engineering neuronal signaling. Prog Brain Res. 2011;194:241–52. https://doi.org/10.1016/B978-0-444-53815-4.00003-0.
Farole A, Jamal BT. A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: a case series. J Oral Maxillofac Surg: Off J Am Assoc Oral Maxillofac Surgeons. 2008;66(10):2058–62. https://doi.org/10.1016/j.joms.2008.06.017.
Finkelstein E, Chang W, Chao P-HG, Gruber D, Minden A, Hung CT, Bulinski JC. Roles of microtubules, cell polarity and adhesion in electric-field-mediated motility of 3T3 fibroblasts. J Cell Sci. 2004;117(8):1533–45. https://doi.org/10.1242/jcs.00986.
Gao T, Huang F, Wang W, Xie Y, Wang B. Interleukin-10 genetically modified clinical-grade mesenchymal stromal cells markedly reinforced functional recovery after spinal cord injury via directing alternative activation of macrophages. Cell Mol Biol Lett. 2022;27(1):27. https://doi.org/10.1186/s11658-022-00325-9.
Georgiou M, Bunting SCJ, Davies HA, Loughlin AJ, Golding JP, Phillips JB. Engineered neural tissue for peripheral nerve repair. Biomaterials. 2013;34(30):7335–43. https://doi.org/10.1016/j.biomaterials.2013.06.025.
Georgiou M, Golding JP, Loughlin AJ, Kingham PJ, Phillips JB. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials. 2015;37:242–51. https://doi.org/10.1016/j.biomaterials.2014.10.009.
Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:e698256. https://doi.org/10.1155/2014/698256.
Grosse G, Lindner G, Schneider P. The effect of the electric field on in vitro cultured nerve tissue. Z Mikrosk-Anat Forsch. 1969;80(2):260–8.
Guo W, Qiu J, Liu J, Liu H. Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci Rep. 2017;7(1):5678. https://doi.org/10.1038/s41598-017-06051-z.
Hardy JG, Cornelison RC, Sukhavasi RC, Saballos RJ, Vu P, Kaplan DL, Schmidt CE. Electroactive tissue scaffolds with aligned pores as instructive platforms for biomimetic tissue engineering. Bioengineering (Basel, Switzerland). 2015;2(1):15–34. https://doi.org/10.3390/bioengineering2010015.
Hronik-Tupaj M, Raja WK, Tang-Schomer M, Omenetto FG, Kaplan DL. Neural responses to electrical stimulation on patterned silk films. J Biomed Mater Res A. 2013;101(9):2559–72. https://doi.org/10.1002/jbm.a.34565.
Huang F, Gao T, Wang W, Wang L, Xie Y, Tai C, Liu S, Cui Y, Wang B. Engineered basic fibroblast growth factor-overexpressing human umbilical cord-derived mesenchymal stem cells improve the proliferation and neuronal differentiation of endogenous neural stem cells and functional recovery of spinal cord injury by activating the PI3K-Akt-GSK-3β signaling pathway. Stem Cell Res Ther. 2021;12(1):468. https://doi.org/10.1186/s13287-021-02537-w.
Huang J, Zhang Y, Lu L, Hu X, Luo Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur J Neurosci. 2013;38(12):3691–701. https://doi.org/10.1111/ejn.12370.
Irfan M, Kim JH, Druzinsky RE, Ravindran S, Chung S. Complement C5aR/LPS-induced BDNF and NGF modulation in human dental pulp stem cells. Sci Rep. 2022;12(1):2042. https://doi.org/10.1038/s41598-022-06110-0.
Jeon I-Y, Chang DW, Kumar NA, Baek J-B, Jeon I-Y, Chang DW, Kumar NA, Baek J-B. Functionalization of carbon nanotubes. In Carbon Nanotubes—Polym Nanocomposites. 2011. https://doi.org/10.5772/18396. (IntechOpen).
Jevans B, James ND, Burnside E, McCann CJ, Thapar N, Bradbury EJ, Burns AJ. Combined treatment with enteric neural stem cells and chondroitinase ABC reduces spinal cord lesion pathology. Stem Cell Res Ther. 2021;12(1):10. https://doi.org/10.1186/s13287-020-02031-9.
Jevans B, McCann CJ, Thapar N, Burns AJ. Transplanted enteric neural stem cells integrate within the developing chick spinal cord: implications for spinal cord repair. J Anat. 2018;233(5):592–606. https://doi.org/10.1111/joa.12880.
Jin H, Zhang Y-T, Yang Y, Wen L-Y, Wang J-H, Xu H-Y, Lai B-Q, Feng B, Che M-T, Qiu X-C, Li Z-L, Wang L-J, Ruan J-W, Jiang B, Zeng X, Deng Q-W, Li G, Ding Y, Zeng Y-S. Electroacupuncture facilitates the integration of neural stem cell-derived neural network with transected rat spinal cord. Stem Cell Reports. 2019;12(2):274–89. https://doi.org/10.1016/j.stemcr.2018.12.015.
Kai D, Tan MJ, Prabhakaran MP, Chan BQY, Liow SS, Ramakrishna S, Loh XJ. Biocompatible electrically conductive nanofibers from inorganic-organic shape memory polymers. Colloids Surf B Biointerfaces. 2016;148:557–65. https://doi.org/10.1016/j.colsurfb.2016.09.035.
Kasper M, Deister C, Beck F, Schmidt CE. Bench-to-bedside lessons learned: commercialization of an acellular nerve graft. Adv Healthcare Mater. 2020;9(16):e2000174. https://doi.org/10.1002/adhm.202000174.
Kasper M, Ellenbogen B, Hardy R, Cydis M, Mojica-Santiago J, Afridi A, Spearman BS, Singh I, Kuliasha CA, Atkinson E, Otto KJ, Judy JW, Rinaldi-Ramos C, Schmidt CE. Development of a magnetically aligned regenerative tissue-engineered electronic nerve interface for peripheral nerve applications. Biomaterials. 2021;279:121212. https://doi.org/10.1016/j.biomaterials.2021.121212.
Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4):457–70. https://doi.org/10.1016/j.addr.2012.09.043.
Kunisaki A, Kodama A, Ishikawa M, Ueda T, Lima MD, Kondo T, Adachi N. Carbon-nanotube yarns induce axonal regeneration in peripheral nerve defect. Sci Rep. 2021;11(1):1. https://doi.org/10.1038/s41598-021-98603-7.
Li ST, Archibald SJ, Krarup C, Madison RD. Peripheral nerve repair with collagen conduits. Clin Mater. 1992;9(3–4):195–200. https://doi.org/10.1016/0267-6605(92)90100-8.
Li Y, Ma Z, Ren Y, Lu D, Li T, Li W, et al. Tissue engineering strategies for peripheral nerve regeneration. Front Neurol. 2021:12. https://doi.org/10.3389/fneur.2021.768267.
Li Y, Neoh KG, Kang E-T. Plasma protein adsorption and thrombus formation on surface functionalized polypyrrole with and without electrical stimulation. J Colloid Interface Sci. 2004;275(2):488–95. https://doi.org/10.1016/j.jcis.2004.02.060.
Liao I-C, Wan H, Qi S, Cui C, Patel P, Sun W, Xu H. Preclinical evaluations of acellular biological conduits for peripheral nerve regeneration. J Tissue Eng. 2013;4:2041731413481036. https://doi.org/10.1177/2041731413481036.
Liau LL, Looi QH, Chia WC, Subramaniam T, Ng MH, Law JX. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020;10:112. https://doi.org/10.1186/s13578-020-00475-3.
Lin Y-X, Li S-H, Huang W-C. Fabrication of soft tissue scaffold-mimicked microelectrode arrays using enzyme-mediated transfer printing. Micromachines. 2021;12(9):1057. https://doi.org/10.3390/mi12091057.
Liu H, Pu Y, Xu Y, Xu H, Liu H, Cheng Y, Xu W, Chen X, Fan J. Olfactory-ensheathing cells promote physiological repair of injured recurrent laryngeal nerves and functional recovery of glottises in dogs. Mol Cell Biochem. 2018;446(1–2):115–25. https://doi.org/10.1007/s11010-018-3279-y.
Lohmeyer JA, Siemers F, Machens H-G, Mailänder P. The clinical use of artificial nerve conduits for digital nerve repair: a prospective cohort study and literature review. J Reconstr Microsurg. 2009;25(1):55–61. https://doi.org/10.1055/s-0028-1103505.
Magaz A, Li X, Gough JE, Blaker JJ. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater Sci Eng C Mater Biol Appl. 2021;119:111632. https://doi.org/10.1016/j.msec.2020.111632.
Marsh SE, Blurton-Jones M. Neural stem cell therapy for neurodegenerative disorders: the role of neurotrophic support. Neurochem Int. 2017;106:94–100. https://doi.org/10.1016/j.neuint.2017.02.006.
Mataliotakis GI, Tsirikos AI. Spinal cord trauma: pathophysiology, classification of spinal cord injury syndromes, treatment principles and controversies. Orthop Trauma. 2016;30(5):440–9. https://doi.org/10.1016/j.mporth.2016.07.006.
Mathot F, Rbia N, Thaler R, Bishop AT, van Wijnen AJ, Shin AY. Introducing human adipose-derived mesenchymal stem cells to AvanceⓇ nerve grafts and NeuraGenⓇ nerve guides. J Plast Reconstr Aesthet Surg: JPRAS. 2020;73(8):1473–81. https://doi.org/10.1016/j.bjps.2020.03.012.
McCaig CD, Zhao M. Physiological electrical fields modify cell behaviour. BioEssays. 1997;19(9):819–26. https://doi.org/10.1002/bies.950190912.
Meek MF, Coert JH. US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann Plast Surg. 2008;60(1):110–6. https://doi.org/10.1097/SAP.0b013e31804d441c.
Mutepfa AR, Hardy JG, Adams CF. Electroactive scaffolds to improve neural stem cell therapy for spinal cord injury. Front Med Technol. 2022;4:693438. https://doi.org/10.3389/fmedt.2022.693438.
Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A, Nitahara-Kasahara Y, Imagawa K, Hirato T, Ueda M, Kimura K, Okada T. Mesenchymal stem cells overexpressing interleukin-10 promote neuroprotection in experimental acute ischemic stroke. Mol Ther Methods Clin Dev. 2017;6:102–11. https://doi.org/10.1016/j.omtm.2017.06.005.
Navissano M, Malan F, Carnino R, Battiston B. Neurotube for facial nerve repair. Microsurgery. 2005;25(4):268–71. https://doi.org/10.1002/micr.20128.
Nectow AR, Marra KG, Kaplan DL. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng B Rev. 2012;18(1):40–50. https://doi.org/10.1089/ten.TEB.2011.0240.
Nejati S, KarimiSoflou R, Khorshidi S, Karkhaneh A. Development of an oxygen-releasing electroconductive in-situ crosslinkable hydrogel based on oxidized pectin and grafted gelatin for tissue engineering applications. Colloids Surf B Biointerfaces. 2020;196:111347. https://doi.org/10.1016/j.colsurfb.2020.111347.
Ng SY, Lee AYW. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528. https://doi.org/10.3389/fncel.2019.00528.
Nune M, Manchineella S, T G, K S N. Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;94:17–25. https://doi.org/10.1016/j.msec.2018.09.014.
Pagella P, Miran S, Neto E, Martin I, Lamghari M, Mitsiadis TA. Human dental pulp stem cells exhibit enhanced properties in comparison to human bone marrow stem cells on neurites outgrowth. FASEB J: Off Publ Fed Am Soc Exp Biol. 2020;34(4):5499–511. https://doi.org/10.1096/fj.201902482R.
Pang Q-M, Chen S-Y, Xu Q-J, Fu S-P, Yang Y-C, Zou W-H, Zhang M, Liu J, Wan W-H, Peng J-C, Zhang T. Neuroinflammation and scarring after spinal cord injury: therapeutic roles of MSCs on inflammation and glial scar. Front Immunol. 2021;12:751021. https://doi.org/10.3389/fimmu.2021.751021.
Peruzzaro ST, Andrews MMM, Al-Gharaibeh A, Pupiec O, Resk M, Story D, Maiti P, Rossignol J, Dunbar GL. Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury. J Neuroinflammation. 2019;16(1):2. https://doi.org/10.1186/s12974-018-1383-2.
Quigley AF, Razal JM, Thompson BC, Moulton SE, Kita M, Kennedy EL, Clark GM, Wallace GG, Kapsa RMI. A conducting-polymer platform with biodegradable fibers for stimulation and guidance of axonal growth. Adv Mater. 2009;21(43):4393–7. https://doi.org/10.1002/adma.200901165.
Rinker B, Zoldos J, Weber RV, Ko J, Thayer W, Greenberg J, Leversedge FJ, Safa B, Buncke G. Use of processed nerve allografts to repair nerve injuries greater than 25 mm in the hand. Ann Plast Surg. 2017;78(6S Suppl 5):S292–5. https://doi.org/10.1097/SAP.0000000000001037.
Rosson GD, Williams EH, Dellon AL. Motor nerve regeneration across a conduit. Microsurgery. 2009;29(2):107–14. https://doi.org/10.1002/micr.20580.
Shinyama K. Mechanical and electrical properties of polylactic acid with aliphatic-aromatic polyester. J Eng. 2018;2018:e6597183. https://doi.org/10.1155/2018/6597183.
Shrestha S, Shrestha BK, Lee J, Joong OK, Kim B-S, Park CH, Kim CS. A conducting neural interface of polyurethane/silk-functionalized multiwall carbon nanotubes with enhanced mechanical strength for neuroregeneration. Mater Sci Eng C Mater Biol Appl. 2019;102:511–23. https://doi.org/10.1016/j.msec.2019.04.053.
Spearman BS, Kuliasha CA, Judy JW, Schmidt CE. Integration of flexible polyimide arrays into soft extracellular matrix-based hydrogel materials for a tissue-engineered electronic nerve interface (TEENI). J Neurosci Methods. 2020;341:108762. https://doi.org/10.1016/j.jneumeth.2020.108762.
Stocco E, Barbon S, Macchi V, Tiengo C, Petrelli L, Rambaldo A, Borean A, Capelli S, Filippi A, Romanato F, Parnigotto PP, Grandi C, De Caro R, Porzionato A. New bioresorbable wraps based on oxidized polyvinyl alcohol and leukocyte-fibrin-platelet membrane to support peripheral nerve neurorrhaphy: Preclinical comparison versus NeuraWrap. Sci Rep. 2019;9(1):17193. https://doi.org/10.1038/s41598-019-53812-z.
Su D, Zhou J, Ahmed KS, Ma Q, Lv G, Chen J. Fabrication and characterization of collagen-heparin-polypyrrole composite conductive film for neural scaffold. Int J Biol Macromol. 2019;129:895–903. https://doi.org/10.1016/j.ijbiomac.2019.02.087.
Suryavanshi JR, Cox C, Osemwengie BO, Jones HB, MacKay BJ. Sutureless repair of a partially transected median nerve using Tisseel glue and Axoguard nerve protector: a case report. Microsurgery. 2020;40(8):896–900. https://doi.org/10.1002/micr.30593.
Tagandurdyyeva NA, Trube MA, Shemyakin IO, Solomitskiy DN, Medvedev GV, Dresvyanina EN, Nashchekina YA, Ivan’kova EM, Dobrovol’skaya IP, Kamalov AM, Sukhorukova EG, Moskalyuk OA, Yudin VE. Properties of resorbable conduits based on poly(l-lactide) nanofibers and chitosan fibers for peripheral nerve regeneration. Polymers. 2023;15(15):15. https://doi.org/10.3390/polym15153323.
Tandon N, Goh B, Marsano A, Chao P-HG, Montouri-Sorrentino C, Gimble J, et al. Alignment and elongation of human adipose-derived stem cells in response to direct-current electrical stimulation. 2009 Ann Int Conf IEEE Eng Med Biol Soc. 2009;6517–21. https://doi.org/10.1109/IEMBS.2009.5333142.
Tomaskovic-Crook E, Zhang P, Ahtiainen A, Kaisvuo H, Lee C-Y, Beirne S, Aqrawe Z, Svirskis D, Hyttinen J, Wallace GG, Travas-Sejdic J, Crook JM. Human neural tissues from neural stem cells using conductive biogel and printed polymer microelectrode arrays for 3D electrical stimulation. Adv Healthcare Mater. 2019;8(15):e1900425. https://doi.org/10.1002/adhm.201900425.
Urbanchek MG, Shim BS, Baghmanli Z, Wei B, Schroeder K, Langhals NB, Miriani RM, Egeland BM, Kipke DR, Martin DC, Cederna PS. Conduction properties of decellularized nerve biomaterials. IFMBE Proc. 2010;32:430–3. https://doi.org/10.1007/978-3-642-14998-6_109.
Wang J, Cheng Y, Chen L, Zhu T, Ye K, Jia C, Wang H, Zhu M, Fan C, Mo X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019;84:98–113. https://doi.org/10.1016/j.actbio.2018.11.032.
Wang J, Tian L, Chen N, Ramakrishna S, Mo X. The cellular response of nerve cells on poly-l-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation. Mater Sci Eng C Mater Biol Appl. 2018;91:715–26. https://doi.org/10.1016/j.msec.2018.06.025.
Wang L, Lu C, Yang S, Sun P, Wang Y, Guan Y, et al. A fully biodegradable and self-electrified device for neuroregenerative medicine. Sci Adv. 2020;6(50):eabc6686. https://doi.org/10.1126/sciadv.abc6686.
Wang S, Guan S, Li W, Ge D, Xu J, Sun C, Liu T, Ma X. 3D culture of neural stem cells within conductive PEDOT layer-assembled chitosan/gelatin scaffolds for neural tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;93:890–901. https://doi.org/10.1016/j.msec.2018.08.054.
Wang S, Guan S, Zhu Z, Li W, Liu T, Ma X. Hyaluronic acid doped-poly(3,4-ethylenedioxythiophene)/chitosan/gelatin (PEDOT-HA/Cs/Gel) porous conductive scaffold for nerve regeneration. Mater Sci Eng C Mater Biol Appl. 2017;71:308–16. https://doi.org/10.1016/j.msec.2016.10.029.
Wang Y, Zhang Y, Zhang Z, Su Y, Wang Z, Dong M, Chen M. An injectable high-conductive bimaterial scaffold for neural stimulation. Colloids Surf B Biointerfaces. 2020;195:111210. https://doi.org/10.1016/j.colsurfb.2020.111210.
Wenjin W, Wenchao L, Hao Z, Feng L, Yan W, Wodong S, Xianqun F, Wenlong D. Electrical stimulation promotes BDNF expression in spinal cord neurons through Ca(2+)- and Erk-dependent signaling pathways. Cell Mol Neurobiol. 2011;31(3):459–67. https://doi.org/10.1007/s10571-010-9639-0.
Wu Q, Xiang Z, Ying Y, Huang Z, Tu Y, Chen M, Ye J, Dou H, Sheng S, Li X, Ying W, Zhu S. Nerve growth factor (NGF) with hypoxia response elements loaded by adeno-associated virus (AAV) combined with neural stem cells improve the spinal cord injury recovery. Cell Death Discov. 2021;7(1):301. https://doi.org/10.1038/s41420-021-00701-y.
Wu Y, Wang L, Guo B, Shao Y, Ma PX. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials. 2016;87:18–31. https://doi.org/10.1016/j.biomaterials.2016.02.010.
Xu C, Guan S, Wang S, Gong W, Liu T, Ma X, Sun C. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;84:32–43. https://doi.org/10.1016/j.msec.2017.11.032.
Xue X, Zhang L, Yin X, Chen X-X, Chen Z-F, Wang C-X, Xiang Y, Liu M-Y, Zhao J-H. Transplantation of neural stem cells preconditioned with high-mobility group box 1 facilitates functional recovery after spinal cord injury in rats. Mol Med Rep. 2020;22(6):4725–33. https://doi.org/10.3892/mmr.2020.11565.
Yang Y, Xu H-Y, Deng Q-W, Wu G-H, Zeng X, Jin H, Wang L-J, Lai B-Q, Li G, Ma Y-H, Jiang B, Ruan J-W, Wang Y-Q, Ding Y, Zeng Y-S. Electroacupuncture facilitates the integration of a grafted TrkC-modified mesenchymal stem cell-derived neural network into transected spinal cord in rats via increasing neurotrophin-3. CNS Neurosci Ther. 2021;27(7):776–91. https://doi.org/10.1111/cns.13638.
Yuan B, Aziz MRF, Li S, Wu J, Li D, Li R-K. An electro-spun tri-component polymer biomaterial with optoelectronic properties for neuronal differentiation. Acta Biomater. 2022;139:82–90. https://doi.org/10.1016/j.actbio.2021.05.036.
Zarei M, Samimi A, Khorram M, Abdi MM, Golestaneh SI. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int J Biol Macromol. 2021;168:175–86. https://doi.org/10.1016/j.ijbiomac.2020.12.031.
Zeng Y, Ding Y, Xu H, Zeng X, Lai B, Li G, Ma Y. Electro-acupuncture and its combination with adult stem cell transplantation for spinal cord injury treatment: a summary of current laboratory findings and a review of literature. CNS Neurosci Ther. 2022;28(5):635–47. https://doi.org/10.1111/cns.13813.
Zhang B, Wang D, Li X, Yang S, Yuan H. NEP1-40-overexpressing neural stem cells enhance axon regeneration by inhibiting Nogo-A/NgR1 signaling pathway. Curr Neurovasc Res. 2021;18(3):271–8. https://doi.org/10.2174/1567202618666210920115716.
Zhao Y, Liang Y, Ding S, Zhang K, Mao H-Q, Yang Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials. 2020;255:120164. https://doi.org/10.1016/j.biomaterials.2020.120164.
Zhou X, Yang A, Huang Z, Yin G, Pu X, Jin J. Enhancement of neurite adhesion, alignment and elongation on conductive polypyrrole-poly(lactide acid) fibers with cell-derived extracellular matrix. Colloids Surf B Biointerfaces. 2017;149:217–25. https://doi.org/10.1016/j.colsurfb.2016.10.014.
Zhou Z, Liu X, Wu W, Park S, Miller Ii AL, Terzic A, Lu L. Effective nerve cell modulation by electrical stimulation of carbon nanotube embedded conductive polymeric scaffolds. Biomater Sci. 2018;6(9):2375–85. https://doi.org/10.1039/c8bm00553b.
Zhu R, Sun Z, Li C, Ramakrishna S, Chiu K, He L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp Neurol. 2019;319:112963. https://doi.org/10.1016/j.expneurol.2019.112963.
Zhukauskas R, Fischer DN, Deister C, Alsmadi NZ, Mercer D. A comparative study of porcine small intestine submucosa and cross-linked bovine type i collagen as a nerve conduit. J Hand Surg Glob Online. 2021;3(5):282–8. https://doi.org/10.1016/j.jhsg.2021.06.006.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by IG, KS and PC.
Corresponding author
Ethics declarations
Ethics Approval
This study does not require ethical approval because neither humans nor animals are involved.
Consent to Participate
A written informed consent statement is not required as it was an observation study.
Consent for Publication
A consent statement is not required as it was an observation study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gulati, I., Satyam, K. & Chandra, P. Electroactive Conduits for Neuroregeneration: A Step Ahead. Regen. Eng. Transl. Med. (2024). https://doi.org/10.1007/s40883-024-00331-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40883-024-00331-7