Abstract
Purpose
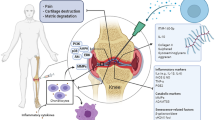
Knee joints are prone to injuries and degenerative diseases; however, current treatments have limited effectiveness in cartilage regeneration. Extracellular vesicles (EVs) derived from MSCs offer potential for promoting cartilage regeneration, especially when associated with hydrogel for controllable delivery. In this context, the systematic review aims to assess the therapeutic potential of hydrogel-loaded EVs in animal models of knee joint disorders.
Material and Methods
This review was conducted from March to June 2023, adhering to the Priority Reporting Items Guidelines for Systematic Reviews and Meta-Analysis (PRISMA) and the SYRCLE Prejudice Risk Tool for Quality Assessment. The selected databases included Embase, Cochrane, Web of Science, PubMed, and Lilacs.
Results
The survey resulted in 344 articles suitable for sorting. After applying pre-defined criteria, 8 studies were selected for inclusion, where the heterogeneity of materials and the absence of standardized protocols for manufacturing EVs-enriched hydrogels are apparent. In the groups treated with EVs-enriched hydrogel, improvements were observed in terms of morphology, with a more organized and smoother structure, as well as an increased number of chondrocytes and more significant deposition of GAG in the treated animals. Furthermore, there was a greater enhancement in immunostaining for COL II, ACAN, and SOX-9, while matrix-degrading factors exhibited a reduction.
Conclusion
Hydrogels loaded with EVs show promise for stimulating cartilage repair and enhancing cell proliferation. The combination of hydrogels and EVs could represent an innovative therapeutic intervention for cartilage repair injuries.
Lay Summary
This systematic review assessed studies on hydrogel-loaded EVs for cartilage repair. Out of 344 articles, 8 were included, highlighting material heterogeneity and a lack of standardized protocols. EVs-enriched hydrogel treatments showed improved morphology, increased chondrocytes, and enhanced GAG deposition. Notably, immunostaining for key markers exhibited enhancement, while matrix-degrading factors decreased. These findings suggest that hydrogels loaded with EVs hold potential for stimulating cartilage repair and promoting cell proliferation. Combining hydrogels with EVs may offer an innovative therapeutic approach for cartilage repair injuries.


Similar content being viewed by others
Data Availability
All data presented in the study are available in the table provided. Any further inquiries may be directed to the corresponding author.
References
Comblain F, Rocasalbas G, Gauthier S, Henrotin Y. Chitosan: A promising polymer for cartilage repair and viscosupplementation. Biomed Mater Eng. 2017;28:S209–15. https://doi.org/10.3233/BME-171643.
Kaderli S, Boulocher C, Pillet E, et al. A novel biocompatible hyaluronic acid-chitosan hybrid hydrogel for osteoarthrosis therapy. Int J Pharm. 2015;483:158–68. https://doi.org/10.1016/j.ijpharm.2015.01.052.
Liu Z, Zhuang Y, Fang L, et al. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact Mater. 2023;22:423–52. https://doi.org/10.1016/j.bioactmat.2022.10.012.
Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. https://doi.org/10.1038/nrrheum.2014.157.
Filardo G, Vannini F, Marcacci M, et al. Matrix-assisted autologous chondrocyte transplantation for cartilage regeneration in osteoarthritic knees: Results and failures at midterm follow-up. Am J Sports Med. 2013;41:95–100. https://doi.org/10.1177/0363546512463675.
Wyszyńska J, Bal-Bocheńska M. Efficacy of high-intensity laser therapy in treating knee osteoarthritis: a first systematic review. Photomed Laser Surg. 2018;36:343–53. https://doi.org/10.1089/pho.2017.4425.
Huang Z, Chen J, Ma J, et al. Effectiveness of low-level laser therapy in patients with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthr Cartil. 2015;23:1437–44. https://doi.org/10.1016/j.joca.2015.04.005.
Wang AT, Zhao M, Feng Y, et al. Multifaceted optimization of MSC-based formulation upon sodium iodoacetate-induced osteoarthritis models by combining advantageous HA/PG hydrogel and fluorescent tracer. Stem Cells Int. 2021;2021. https://doi.org/10.1155/2021/8827212
Yan X, Yan F, Mohammed HAG, Liu O. Maxillofacial-derived mesenchymal stem cells: characteristics and progress in tissue regeneration. Stem Cells Int. 2021;2021. https://doi.org/10.1155/2021/5516521
Tao SC, Guo SC, Zhang CQ. Platelet-derived extracellular vesicles: An emerging therapeutic approach. Int J Biol Sci. 2017;13:828–34. https://doi.org/10.7150/ijbs.19776.
Ni Z, Kuang L, Chen H, et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10. https://doi.org/10.1038/s41419-019-1739-2
Couch Y, Buzàs EI, Di Vizio D, et al. A brief history of nearly EV-erything - The rise and rise of extracellular vesicles. J Extracell Vesicles. 2021;10:e12144. https://doi.org/10.1002/jev2.12144.
Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell. 2019;177:428-445.e18. https://doi.org/10.1016/j.cell.2019.02.029.
Akers JC, Gonda D, Kim R, et al. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. https://doi.org/10.1007/s11060-013-1084-8.
El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. https://doi.org/10.1038/nrd3978.
Clancy JW, Schmidtmann M, D’Souza-Schorey C. The ins and outs of microvesicles. FASEB bioAdvances. 2021;3:399–406. https://doi.org/10.1096/fba.2020-00127.
Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–80. https://doi.org/10.1038/nri3607.
Zhang S, Teo KYW, Chuah SJ, et al. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. https://doi.org/10.1016/j.biomaterials.2019.02.006.
Zhang S, Chu WC, Lai RC, et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil. 2016;24:2135–40. https://doi.org/10.1016/j.joca.2016.06.022.
Wu J, Kuang L, Chen C, et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. https://doi.org/10.1016/j.biomaterials.2019.03.022.
Zhu Y, Wang Y, Zhao B, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8:1–11. https://doi.org/10.1186/s13287-017-0510-9.
Vilela CA, Correia C, Oliveira JM, et al. Cartilage repair using hydrogels: a critical review of in vivo experimental designs. ACS Biomater Sci Eng. 2015;1:726–39. https://doi.org/10.1021/acsbiomaterials.5b00245.
Zhang K, Zhao X, Chen X, et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for Hindlimb ischemia treatment. ACS Appl Mater Interfaces. 2018;10:30081–91. https://doi.org/10.1021/acsami.8b08449.
Mol EA, Lei Z, Roefs MT, et al. Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv Healthc Mater. 2019;8. https://doi.org/10.1002/adhm.201900847
Amengual-Tugores AM, Ráez-Meseguer C, Forteza-Genestra MA, et al. Extracellular vesicle-based hydrogels for wound healing applications. Int J Mol Sci. 2023;24. https://doi.org/10.3390/ijms24044104
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Hooijmans CR, Rovers MM, De Vries RBM, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9. https://doi.org/10.1186/1471-2288-14-43.
Hu H, Dong L, Bu Z, et al. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J Extracell Vesicles. 2020;9. https://doi.org/10.1080/20013078.2020.1778883
Cheng J, Rong G, Wang Z, et al. ECM-mimicking hydrogels loaded with bone mesenchymal stem cell-derived exosomes for the treatment of cartilage defects. Evid-Based Complement Altern Med 2022;2022. https://doi.org/10.1155/2022/3450672
Tao SC, Huang JY, Gao Y, et al. Small extracellular vesicles in combination with sleep-related circRNA3503: A targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact Mater. 2021;6:4455–69. https://doi.org/10.1016/j.bioactmat.2021.04.031.
Yang Y, Zhu Z, Gao R, et al. Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic acid/PEG hydrogel for osteoarthritis improvement. Acta Biomater. 2021;128:163–74. https://doi.org/10.1016/j.actbio.2021.04.003.
Zhao Y, Zhao X, Xu H, et al. Wharton’s jelly MSC-derived extracellular vehicles—loaded hyaluronic acid-alginate adhesives for treatment of osteoarthritis. J Mater Sci Technol. 2023;142:240–52. https://doi.org/10.1016/j.jmst.2022.09.061.
Liu X, Yang Y, Li Y, et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430–8. https://doi.org/10.1039/c7nr00352h.
Sun Y, Zhao J, Wu Q, et al. Chondrogenic primed extracellular vesicles activate miR-455/SOX11/FOXO axis for cartilage regeneration and osteoarthritis treatment. npj Regen Med. 2022;7. https://doi.org/10.1038/s41536-022-00250-7
Pang L, Jin H, Lu Z, et al. Treatment with mesenchymal stem cell-derived nanovesicle-containing gelatin methacryloyl hydrogels alleviates osteoarthritis by modulating chondrogenesis and macrophage polarization. Adv Healthc Mater. 2023;2300315:2300315. https://doi.org/10.1002/adhm.202300315.
Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10:1–13. https://doi.org/10.1186/s13287-019-1445-0.
Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Engineering Part B: Reviews. 2010;16(1):105–15. https://doi.org/10.1089/ten.TEB.2009.0452.
Meng X, Ziadlou R, Grad S, et al. Animal Models of osteochondral defect for testing biomaterials. Biochem Res Int. 2020;2020. https://doi.org/10.1155/2020/9659412
Li S, Stöckl S, Lukas C, et al. hBMSC-Derived extracellular vesicles attenuate IL-1β-induced catabolic effects on OA-chondrocytes by regulating pro-inflammatory signaling pathways. Front Bioeng Biotechnol. 2020;8:1–14. https://doi.org/10.3389/fbioe.2020.603598.
He L, He T, Xing J, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11:1–15. https://doi.org/10.1186/s13287-020-01781-w.
Qiu M, Liu D, Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. https://doi.org/10.1016/j.lfs.2020.118987.
Liu YJ, Wang C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun Signal. 2023;21:77. https://doi.org/10.1186/s12964-023-01103-6.
Jankovičová J, Sečová P, Michalková K, Antalíková J. Tetraspanins, more than markers of extracellular vesicles in reproduction. Int J Mol Sci. 2020;21:1–30.
Jin Y, Koh RH, Kim SH, et al. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater Sci Eng C. 2020;115:111096. https://doi.org/10.1016/j.msec.2020.111096.
Liu Y, Peng L, Li L, et al. 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials. 2021;279:121216. https://doi.org/10.1016/j.biomaterials.2021.121216.
Hong H, Seo YB, Kim DY, et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232. https://doi.org/10.1016/j.biomaterials.2019.119679
Tan B, Huang L, Wu Y, Liao J. Advances and trends of hydrogel therapy platform in localized tumor treatment: A review. J Biomed Mater Res - Part A. 2021;109:404–25. https://doi.org/10.1002/jbm.a.37062.
Zhang Y, Liu M, Pei R. An in situ gelling BMSC-laden collagen/silk fibroin double network hydrogel for cartilage regeneration. Mater Adv. 2021;2:4733–42. https://doi.org/10.1039/d1ma00285f.
Vonk LA, van Dooremalen SFJ, Liv N, et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8:906–20. https://doi.org/10.7150/thno.20746.
Li Z, Li M, Xu P, et al. Compositional variation and functional mechanism of exosomes in the articular microenvironment in knee osteoarthritis. Cell Transplant. 2020;29:1–10. https://doi.org/10.1177/0963689720968495.
Sun Y, You Y, Jiang W, et al. 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics. 2019;9:6949–61. https://doi.org/10.7150/thno.38061.
Funding
The authors gratefully acknowledge FAPESP (Grant No. 2022/04816–4) for funding support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection and data analysis were performed by HGM, LCSS and CCSM. The analyses of the levels of evidence and methodological quality of the included studies were performed by JRP. Authors HGM, MB, LCSS, BSS, CCSM, and ACMR were responsible for study writing and review. Also, LA, DAR, AMR assisted in the review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The research was not submitted to an ethics committee, as it was a review study.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia-Motta, H., Bonifacio, M., Martignago, C.C.S. et al. Hydrogels Loaded with Mesenchymal Stem Cells Extracellular Vesicles for Treating Knee Joint Disorders: A Systematic Review. Regen. Eng. Transl. Med. (2023). https://doi.org/10.1007/s40883-023-00326-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40883-023-00326-w